Consider the isoelectronic ions F- and Na+. (b) Using Equation 7.1 and assuming that core electrons contribute 1.00 and valence electrons contribute 0.00 to the screening constant, S, calculate Zeff for the 2p electrons in both ions.
Ch.7 - Periodic Properties of the Elements

Brown14th EditionChemistry: The Central ScienceISBN: 9780134414232Not the one you use?Change textbook
Chapter 7, Problem 32
Consider the isoelectronic ions Cl- and K+. (b) Using Equation 7.1 and assuming that core electrons contribute 1.00 and valence electrons contribute nothing to the screening constant, S, calculate Zeff for these two ions. (c) Repeat this calculation using Slater’s rules to estimate the screening constant, S.
 Verified step by step guidance
Verified step by step guidance1
Step 1: Understand the concept of effective nuclear charge (Z_{eff}). It is the net positive charge experienced by an electron in a multi-electron atom. The formula is Z_{eff} = Z - S, where Z is the atomic number and S is the screening constant.
Step 2: For part (b), calculate Z_{eff} using the assumption that core electrons contribute 1.00 and valence electrons contribute nothing to the screening constant, S. Identify the number of core electrons for Cl^- and K^+.
Step 3: For Cl^-, the atomic number (Z) is 17. Determine the number of core electrons and calculate S. Then, use Z_{eff} = Z - S to find the effective nuclear charge for Cl^-.
Step 4: For K^+, the atomic number (Z) is 19. Determine the number of core electrons and calculate S. Then, use Z_{eff} = Z - S to find the effective nuclear charge for K^+.
Step 5: For part (c), apply Slater's rules to estimate the screening constant, S, for both Cl^- and K^+. Slater's rules provide a more detailed method to calculate S by considering different contributions from electrons in various shells. Use these rules to recalculate Z_{eff} for both ions.
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Effective Nuclear Charge (Zeff)
Effective Nuclear Charge (Zeff) is the net positive charge experienced by an electron in a multi-electron atom. It accounts for the actual nuclear charge (the number of protons) minus the shielding effect of other electrons. This concept is crucial for understanding how strongly an electron is attracted to the nucleus, influencing atomic size and ionization energy.
Recommended video:
Guided course
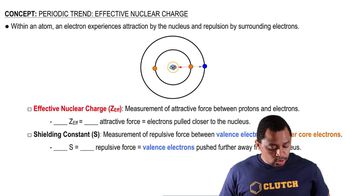
Effective Nuclear Charge
Screening Constant (S)
The screening constant (S) quantifies the extent to which inner electrons shield outer electrons from the full effect of the nuclear charge. In the context of isoelectronic ions, calculating S helps determine how much the core electrons reduce the effective nuclear charge felt by the valence electrons. This is essential for accurately calculating Zeff.
Recommended video:
Guided course

Equilibrium Constant K
Slater's Rules
Slater's Rules provide a systematic method for estimating the screening constant (S) based on the arrangement of electrons in an atom. These rules assign different contributions to electrons based on their principal quantum number and whether they are in the same group or shell. Using Slater's Rules allows for a more refined calculation of Zeff, particularly in complex ions.
Recommended video:
Guided course
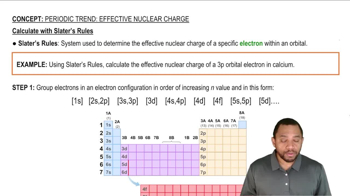
Effective Nuclear Charge Calculation with Slater's Rules
Related Practice
Textbook Question
Textbook Question
Consider the isoelectronic ions F- and Na+. (c) Repeat this calculation using Slater’s rules to estimate the screening constant, S.
Textbook Question
Consider the isoelectronic ions F- and Na+. (d) For isoelectronic ions, how are effective nuclear charge and ionic radius related?
Textbook Question
Consider S, Cl, and K and their most common ions. (a) List the atoms in order of increasing size.
1
views
Textbook Question
Consider S, Cl, and K and their most common ions. (b) List the ions in order of increasing size. (c) Explain any differences in the orders of the atomic and ionic sizes.
Textbook Question
Arrange each of the following sets of atoms and ions, in orderof increasing size: (a) Pb, Pb2+, Pb4+
