Use data from Appendix C, Figure 7.10, and Figure 7.12 to calculate the lattice energy of RbCl.
Ch.7 - Periodic Properties of the Elements

Brown14th EditionChemistry: The Central ScienceISBN: 9780134414232Not the one you use?Change textbook
Chapter 7, Problem 31b
Consider the isoelectronic ions F- and Na+. (b) Using Equation 7.1 and assuming that core electrons contribute 1.00 and valence electrons contribute 0.00 to the screening constant, S, calculate Zeff for the 2p electrons in both ions.
 Verified step by step guidance
Verified step by step guidance1
Identify the isoelectronic ions: F^- and Na^+ both have the same number of electrons, which is 10.
Determine the atomic numbers: Fluorine (F) has an atomic number of 9, and Sodium (Na) has an atomic number of 11.
Use the formula for effective nuclear charge, Z_{eff} = Z - S, where Z is the atomic number and S is the screening constant.
Calculate the screening constant, S, for each ion. Since core electrons contribute 1.00 and valence electrons contribute 0.00, identify the core and valence electrons for each ion. For both ions, the core electrons are the 1s^2 electrons, contributing 2.00 to S.
Substitute the values into the Z_{eff} formula for each ion: For F^-, Z_{eff} = 9 - 2.00; for Na^+, Z_{eff} = 11 - 2.00.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
7mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Isoelectronic Species
Isoelectronic species are atoms or ions that have the same number of electrons and, therefore, the same electronic configuration. In this case, F- and Na+ both have 10 electrons, making them isoelectronic. Understanding this concept is crucial for comparing their effective nuclear charges (Zeff) since they experience similar electron-electron interactions despite differing nuclear charges.
Recommended video:
Guided course
Amphoteric Species
Effective Nuclear Charge (Zeff)
Effective nuclear charge (Zeff) is the net positive charge experienced by an electron in a multi-electron atom. It accounts for the actual nuclear charge minus the shielding effect of other electrons. Calculating Zeff helps in understanding how strongly the nucleus attracts its electrons, which is essential for predicting chemical behavior and properties of ions like F- and Na+.
Recommended video:
Guided course
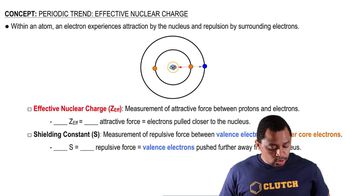
Effective Nuclear Charge
Screening Constant (S)
The screening constant (S) quantifies the extent to which inner electrons shield outer electrons from the full effect of the nuclear charge. In the context of the question, core electrons contribute a value of 1.00 to S, while valence electrons contribute 0.00. This distinction is important for accurately calculating Zeff for the 2p electrons in the ions, as it directly influences the perceived nuclear charge experienced by these electrons.
Recommended video:
Guided course

Equilibrium Constant K
Related Practice
Textbook Question
Textbook Question
Which neutral atom is isoelectronic with each of the following ions? Ga3+, Zr4+, Mn7+, I−, Pb2+.
4
views
Textbook Question
Some ions do not have a corresponding neutral atom that has the same electron configuration. For each of the following ions, identify the neutral atom that has the same number of electrons and determine if this atom has the same electron configuration. (a) CI−, (b) Sc3+, (c) Fe2+, (d) Zn2+, (e) Sn4+.
1
views
Textbook Question
Consider the isoelectronic ions F- and Na+. (c) Repeat this calculation using Slater’s rules to estimate the screening constant, S.
Textbook Question
Consider the isoelectronic ions F- and Na+. (d) For isoelectronic ions, how are effective nuclear charge and ionic radius related?
