Textbook Question
Consider the isoelectronic ions F- and Na+. (c) Repeat this calculation using Slater’s rules to estimate the screening constant, S.

 Verified step by step guidance
Verified step by step guidance
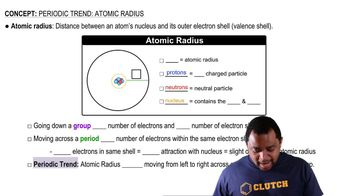

Consider the isoelectronic ions F- and Na+. (c) Repeat this calculation using Slater’s rules to estimate the screening constant, S.
Consider the isoelectronic ions F- and Na+. (d) For isoelectronic ions, how are effective nuclear charge and ionic radius related?
Consider S, Cl, and K and their most common ions. (b) List the ions in order of increasing size. (c) Explain any differences in the orders of the atomic and ionic sizes.
Provide a brief explanation for each of the following: (a) Cl- is larger than Ar. (b) P3- is larger than S2-. (c) K+ is larger than Na+. (d) F- is larger than F.