 Back
BackProblem 45b
The structural formulas of the compounds n-butane and isobutane are shown below. (b) Determine the empirical formula of each.

Problem 45c
The structural formulas of the compounds n-butane and isobutane are shown below. (c) Which formulas—empirical, molecular, or structural—allow you determine these are different compounds?

Problem 46a
Ball-and-stick representations of benzene, a colorless liquid often used in organic chemistry reactions, and acetylene, a gas used as a fuel for high-temperature welding, are shown below. (a) Determine the molecular formula of each.

Problem 47a
What are the molecular and empirical formulas for each of the following compounds? Write the molecular formula for the following compound.

Problem 48
Two substances have the same molecular and empirical formulas. Does this mean that they must be the same compound?
Problem 49a
Write the empirical formula corresponding to each of the following molecular formulas: (a) Al2Br6
Problem 49b
Write the empirical formula corresponding to each of the following molecular formulas: (b) C8H10
Problem 49c
Write the empirical formula corresponding to each of the following molecular formulas: (c) C4H8O2
Problem 49d,e,f
Write the empirical formula corresponding to each of the following molecular formulas: (d) P4O10 (e) C6H4Cl2 (f) B3N3H6.
Problem 50a
Determine the molecular and empirical formulas of the following: (a) the organic solvent benzene, which has six carbon atoms and six hydrogen atoms
Problem 50b
Determine the molecular and empirical formulas of the following: (b) the compound silicon tetrachloride, which has a silicon atom and four chlorine atoms and is used in the manufacture of computer chips
Problem 51
How many hydrogen atoms are in each of the following: (a) C2H5OH? (b) Ca(C2H5COO)2? (c) (NH4)3PO4?
Problem 52a
How many of the indicated atoms are represented by each chemical formula: (a) carbon atoms in C4H9COOCH3
Problem 52b,c
How many of the indicated atoms are represented by each chemical formula: (b) oxygen atoms in Ca(ClO3)2 (c) hydrogen atoms in (NH4)2HPO4?
Problem 53
Write the molecular and structural formulas for the compounds represented by the following molecular models:

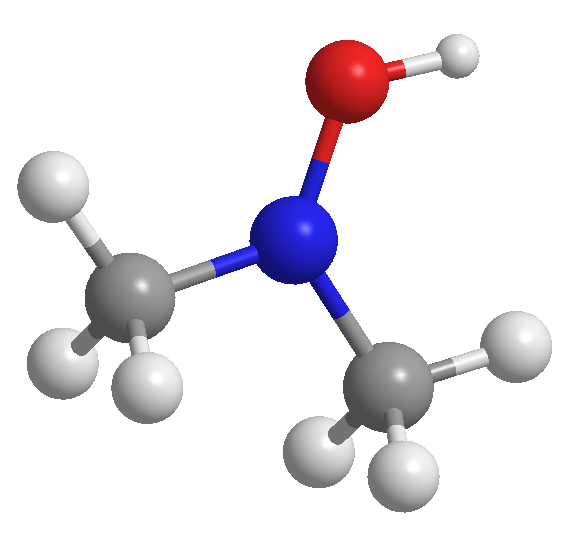
Problem 54
Write the molecular and structural formulas for the compounds represented by the following models:
Problem 55
Fill in the gaps in the following table: Symbol 59Co3+ Protons 34 76 80 Neutrons 46 116 120 Electrons 36 78 Net charge 2+
Problem 56
Fill in the gaps in the following table: Symbol 133Cs+ Protons 35 15 Neutrons 46 16 30 Electrons 18 20 Net charge 1- 5+
Problem 57
Each of the following elements is capable of forming an ion in chemical reactions. By referring to the periodic table, predict the charge of the most stable ion of each: (a) Be (b) Rb (c) As (d) In (e) At.
Problem 58a,b,c,d
Using the periodic table, predict the charge of the most stable ion of the following elements: (a) Li (b) Ba (c) Po (d) I
Problem 58e
Using the periodic table, predict the charge of the most stable ion of the following elements: (e) Sb.
Problem 59a,b
Using the periodic table to guide you, predict the chemical formula and name of the compound formed by the following elements: (a) Ga and F (b) Li and H
Problem 59c
Using the periodic table to guide you, predict the chemical formula and name of the compound formed by the following elements: (c) Al and I
Problem 59d
Using the periodic table to guide you, predict the chemical formula and name of the compound formed by the following elements: (d) K and S.
Problem 60b
The most common charge associated with selenium is 2-. Indicate the chemical formulas you would expect for compounds formed between selenium and (b) lithium
Problem 61a
Predict the chemical formulas of the ionic compound formed by (a) Fe3+ and OH-
Problem 61b
Predict the chemical formulas of the ionic compound formed by (b) Cs+ and NO3
Problem 61c
Predict the chemical formulas of the ionic compound formed by (c) V2+ and CH3COO-
Problem 61d
Predict the chemical formulas of the ionic compound formed by (d) Li+ and PO43-
Problem 61e
Predict the chemical formulas of the ionic compound formed by (e) In3+ and O2-.
