Textbook Question
Write the empirical formula corresponding to each of the following molecular formulas: (a) Al2Br6

 Verified step by step guidance
Verified step by step guidance


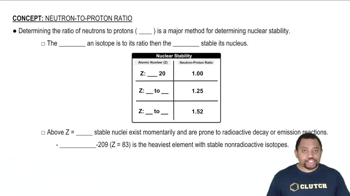
Write the empirical formula corresponding to each of the following molecular formulas: (a) Al2Br6
Write the empirical formula corresponding to each of the following molecular formulas: (b) C8H10
Write the empirical formula corresponding to each of the following molecular formulas: (c) C4H8O2
Determine the molecular and empirical formulas of the following: (a) the organic solvent benzene, which has six carbon atoms and six hydrogen atoms
Determine the molecular and empirical formulas of the following: (b) the compound silicon tetrachloride, which has a silicon atom and four chlorine atoms and is used in the manufacture of computer chips
How many hydrogen atoms are in each of the following: (a) C2H5OH? (b) Ca(C2H5COO)2? (c) (NH4)3PO4?