Textbook Question
Using the periodic table to guide you, predict the chemical formula and name of the compound formed by the following elements: (a) Ga and F (b) Li and H

 Verified step by step guidance
Verified step by step guidance
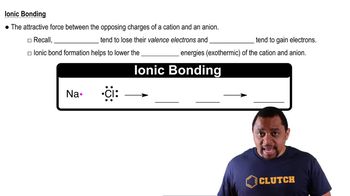

Using the periodic table to guide you, predict the chemical formula and name of the compound formed by the following elements: (a) Ga and F (b) Li and H
Using the periodic table to guide you, predict the chemical formula and name of the compound formed by the following elements: (c) Al and I
Using the periodic table to guide you, predict the chemical formula and name of the compound formed by the following elements: (d) K and S.
Predict the chemical formulas of the ionic compound formed by (a) Fe3+ and OH-
Predict the chemical formulas of the ionic compound formed by (b) Cs+ and NO3
Predict the chemical formulas of the ionic compound formed by (c) V2+ and CH3COO-