Complete the table by filling in the formula for the ionic compound formed by each pair of cations and anions, as shown for the first pair. Ion Na+ Ca2+ Fe2+ Al3+ O2- Na2O NO3- SO42- AsO43-
Ch.2 - Atoms, Molecules, and Ions

Brown14th EditionChemistry: The Central ScienceISBN: 9780134414232Not the one you use?Change textbook
Chapter 2, Problem 65b,c,d
Predict whether each of the following compounds is molecular or ionic: (b) CH3OCH3 (c) Mg(NO3)2 (d) H2S
 Verified step by step guidance
Verified step by step guidance1
Step 1: Understand the difference between molecular and ionic compounds. Molecular compounds are formed by covalent bonds between nonmetals, while ionic compounds are formed by the electrostatic attraction between cations and anions, typically involving a metal and a nonmetal.
Step 2: Identify the elements present in the compound CH3OCH3. This compound consists of carbon (C), hydrogen (H), and oxygen (O).
Step 3: Determine the types of elements involved. Carbon, hydrogen, and oxygen are all nonmetals.
Step 4: Consider the bonding in the compound. Since all the elements are nonmetals, they are likely to form covalent bonds with each other.
Step 5: Conclude that CH3OCH3 is a molecular compound because it is composed of nonmetals bonded covalently.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
2mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Molecular Compounds
Molecular compounds are formed when two or more nonmetals share electrons through covalent bonds. They typically have low melting and boiling points and can exist in various states (solid, liquid, gas) at room temperature. The presence of specific elements, such as carbon and hydrogen, often indicates a molecular compound.
Recommended video:
Guided course
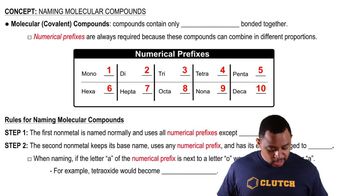
Naming Molecular Compounds
Ionic Compounds
Ionic compounds are formed when metals transfer electrons to nonmetals, resulting in the formation of charged ions that attract each other due to electrostatic forces. These compounds usually have high melting and boiling points and are often soluble in water. The presence of a metal and a nonmetal in a compound typically suggests it is ionic.
Recommended video:
Guided course

Ionic Compounds Naming
Chemical Structure and Bonding
The chemical structure of a compound determines its classification as molecular or ionic. In the case of CH3OCH3 (dimethyl ether), the structure consists of covalent bonds between carbon, hydrogen, and oxygen atoms, indicating it is a molecular compound. Understanding the arrangement of atoms and the types of bonds formed is crucial for predicting the nature of a compound.
Recommended video:
Guided course
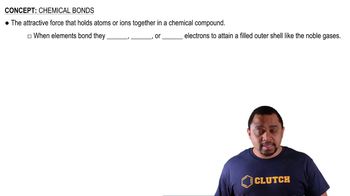
Chemical Bonds
Related Practice
Textbook Question
Textbook Question
Complete the table by filling in the formula for the ionic compound formed by each pair of cations and anions, as shown for the first pair. Ion K+ NH4+ Mg2+ Fe3+ Cl- KCl OH- CO32- PO43-
Complete the first row of the table.
Complete the second row of the table.
Complete the third row of the table.
Complete the fourth row of the table.
Textbook Question
Predict whether each of the following compounds is molecular or ionic: (a) HClO4
Textbook Question
Predict whether each of the following compounds is molecular or ionic: (e) TiCl4
Textbook Question
Predict whether each of the following compounds is molecular or ionic: (f) K2O2 (g) PCl5
Textbook Question
Predict whether each of the following compounds is molecular or ionic: (h) P(OH)3.
