Complete the table by filling in the formula for the ionic compound formed by each pair of cations and anions, as shown for the first pair. Ion Na+ Ca2+ Fe2+ Al3+ O2- Na2O NO3- SO42- AsO43-
Ch.2 - Atoms, Molecules, and Ions

Brown14th EditionChemistry: The Central ScienceISBN: 9780134414232Not the one you use?Change textbook
Chapter 2, Problem 65a
Predict whether each of the following compounds is molecular or ionic: (a) HClO4
 Verified step by step guidance
Verified step by step guidance1
Identify the types of elements involved in the compound HClO_4. Hydrogen (H) and chlorine (Cl) are nonmetals, and oxygen (O) is also a nonmetal.
Recall that molecular compounds are typically formed between nonmetals, while ionic compounds are formed between metals and nonmetals.
Consider the nature of the bonds in HClO_4. Since it consists of nonmetals, it is likely to form covalent bonds, which are characteristic of molecular compounds.
Recognize that HClO_4 is known as perchloric acid, which is a common example of a molecular compound.
Conclude that HClO_4 is a molecular compound because it is composed entirely of nonmetals and forms covalent bonds.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
2mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Ionic vs. Molecular Compounds
Ionic compounds are formed from the electrostatic attraction between positively and negatively charged ions, typically involving a metal and a nonmetal. In contrast, molecular compounds consist of molecules formed by covalent bonds, where atoms share electrons, usually involving nonmetals. Understanding the distinction helps in predicting the properties and behaviors of different compounds.
Recommended video:
Guided course

Ionic Compounds Naming
Electronegativity
Electronegativity is a measure of an atom's ability to attract and hold onto electrons in a chemical bond. In a compound, if there is a significant difference in electronegativity between the bonded atoms, the bond is likely ionic. If the difference is small, the bond is typically covalent, indicating a molecular compound.
Recommended video:
Guided course
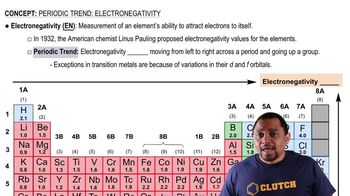
Electronegativity Trends
Common Molecular and Ionic Compounds
Certain compounds are commonly recognized as either ionic or molecular based on their composition. For example, compounds like HClO4 (perchloric acid) are molecular because they consist of nonmetals and exhibit covalent bonding. Familiarity with common examples aids in quickly classifying new compounds based on their chemical formulas.
Recommended video:
Guided course

Ionic Compounds Naming
Related Practice
Textbook Question
Textbook Question
Predict the chemical formulas of the compounds formed by the following pairs of ions: (e) Ti4+ and O2-.
Textbook Question
Complete the table by filling in the formula for the ionic compound formed by each pair of cations and anions, as shown for the first pair. Ion K+ NH4+ Mg2+ Fe3+ Cl- KCl OH- CO32- PO43-
Complete the first row of the table.
Complete the second row of the table.
Complete the third row of the table.
Complete the fourth row of the table.
Textbook Question
Predict whether each of the following compounds is molecular or ionic: (b) CH3OCH3 (c) Mg(NO3)2 (d) H2S
Textbook Question
Predict whether each of the following compounds is molecular or ionic: (e) TiCl4
Textbook Question
Predict whether each of the following compounds is molecular or ionic: (f) K2O2 (g) PCl5
