Predict the chemical formulas of the compounds formed by the following pairs of ions: (d) Cd2+ and CO32-
Ch.2 - Atoms, Molecules, and Ions

Brown14th EditionChemistry: The Central ScienceISBN: 9780134414232Not the one you use?Change textbook
Chapter 2, Problem 64
Complete the table by filling in the formula for the ionic compound formed by each pair of cations and anions, as shown for the first pair. Ion Na+ Ca2+ Fe2+ Al3+ O2- Na2O NO3- SO42- AsO43-
 Verified step by step guidance
Verified step by step guidance1
1. To form an ionic compound, the total positive charge from the cations must balance the total negative charge from the anions. This is because ionic compounds are neutral overall. The subscript in the formula for each ion indicates the charge.
2. For the pair of Ca2+ and O2-, the charges are already balanced. Therefore, the formula for the ionic compound formed by these ions is CaO.
3. For the pair of Fe2+ and O2-, the charges are also balanced. Therefore, the formula for the ionic compound formed by these ions is FeO.
4. For the pair of Al3+ and O2-, the charges are not balanced. To balance the charges, we need two Al3+ ions for every three O2- ions. Therefore, the formula for the ionic compound formed by these ions is Al2O3.
5. Repeat this process for the remaining pairs of ions. Remember, the goal is to balance the total positive charge with the total negative charge.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
1mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Ionic Compounds
Ionic compounds are formed through the electrostatic attraction between cations (positively charged ions) and anions (negatively charged ions). The overall charge of the compound must be neutral, meaning the total positive charge from cations must balance the total negative charge from anions. This principle is essential for determining the correct formula of the compound.
Recommended video:
Guided course

Ionic Compounds Naming
Charge Balance
Charge balance is a fundamental concept in forming ionic compounds, where the sum of the charges from cations and anions must equal zero. For example, a Ca2+ cation requires two O2- anions to achieve neutrality, resulting in the formula CaO. Understanding how to balance these charges is crucial for writing correct chemical formulas.
Recommended video:
Guided course
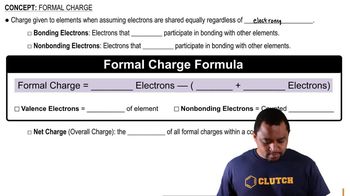
Formal Charge
Polyatomic Ions
Polyatomic ions are ions composed of two or more atoms that carry a net charge. Examples include nitrate (NO3-) and sulfate (SO42-). When forming ionic compounds, the presence of polyatomic ions requires careful consideration of their charge and how they combine with cations to ensure overall charge neutrality in the resulting formula.
Recommended video:
Guided course
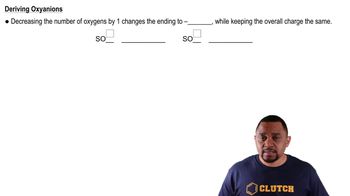
Polyatomic Ion Variations
Related Practice
Textbook Question
Textbook Question
Predict the chemical formulas of the compounds formed by the following pairs of ions: (e) Ti4+ and O2-.
Textbook Question
Complete the table by filling in the formula for the ionic compound formed by each pair of cations and anions, as shown for the first pair. Ion K+ NH4+ Mg2+ Fe3+ Cl- KCl OH- CO32- PO43-
Complete the first row of the table.
Complete the second row of the table.
Complete the third row of the table.
Complete the fourth row of the table.
Textbook Question
Predict whether each of the following compounds is molecular or ionic: (a) HClO4
Textbook Question
Predict whether each of the following compounds is molecular or ionic: (b) CH3OCH3 (c) Mg(NO3)2 (d) H2S
Textbook Question
Predict whether each of the following compounds is molecular or ionic: (e) TiCl4
