Textbook Question
Predict whether each of the following compounds is molecular or ionic: (a) HClO4

 Verified step by step guidance
Verified step by step guidance

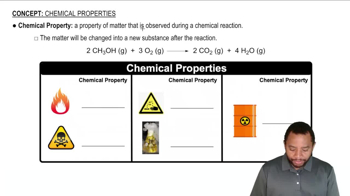
Predict whether each of the following compounds is molecular or ionic: (a) HClO4
Predict whether each of the following compounds is molecular or ionic: (b) CH3OCH3 (c) Mg(NO3)2 (d) H2S
Predict whether each of the following compounds is molecular or ionic: (e) TiCl4
Predict whether each of the following compounds is molecular or ionic: (h) P(OH)3.
Which of the following are ionic, and which are molecular? (a) PF5
Which of the following are ionic, and which are molecular? (a) PF5