Rainwater is acidic because CO21g2 dissolves in the water, creating carbonic acid, H2CO3. If the rainwater is too acidic, it will react with limestone and seashells (which are principally made of calcium carbonate, CaCO3). Calculate the concentrations of carbonic acid, bicarbonate ion 1HCO3-2 and carbonate ion 1CO32 - 2 that are in a raindrop that has a pH of 5.60, assuming that the sum of all three species in the raindrop is 1.0 * 10-5 M.
Ch.17 - Additional Aspects of Aqueous Equilibria

Brown14th EditionChemistry: The Central ScienceISBN: 9780134414232Not the one you use?Change textbook
Chapter 17, Problem 87
Equal quantities of 0.010 M solutions of an acid HA and a base B are mixed. The pH of the resulting solution is 9.2. (a) Write the chemical equation and equilibrium-constant expression for the reaction between HA and B. (b) If Ka for HA is 8.0 × 10⁻⁵, what is the value of the equilibrium constant for the reaction between HA and B?
 Verified step by step guidance
Verified step by step guidance1
Step 1: Write the balanced chemical equation for the reaction between the acid HA and the base B. The general reaction is HA + B ⇌ A⁻ + BH⁺.
Step 2: Write the equilibrium constant expression for the reaction. The equilibrium constant (K) is given by the expression K = [A⁻][BH⁺] / [HA][B].
Step 3: Recognize that the pH of the solution is 9.2, which indicates that the solution is basic. This suggests that the base B is stronger than the acid HA.
Step 4: Use the given pH to find the concentration of OH⁻ ions in the solution. Since pH + pOH = 14, calculate pOH = 14 - 9.2, and then find [OH⁻] using [OH⁻] = 10^(-pOH).
Step 5: Use the relationship between the equilibrium constants of the acid and base reactions. The equilibrium constant for the reaction between HA and B can be found using the formula K = Kw / Ka, where Kw is the ion-product constant of water (1.0 × 10⁻¹⁴ at 25°C) and Ka is the acid dissociation constant for HA.
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Acid-Base Reactions
Acid-base reactions involve the transfer of protons (H⁺ ions) between an acid and a base. In this context, HA represents the acid, which donates a proton, while B represents the base, which accepts the proton. The resulting solution's pH indicates the balance between the concentrations of H⁺ and OH⁻ ions, reflecting the extent of the reaction.
Recommended video:
Guided course

Acid-Base Reaction
Equilibrium Constant (K)
The equilibrium constant (K) quantifies the ratio of the concentrations of products to reactants at equilibrium for a given reaction. For the reaction between HA and B, K can be expressed in terms of the concentrations of the products and reactants. Understanding K is crucial for predicting the direction of the reaction and the extent to which reactants are converted to products.
Recommended video:
Guided course

Equilibrium Constant K
Dissociation Constant (Ka)
The dissociation constant (Ka) measures the strength of an acid in solution, indicating how well it donates protons. A higher Ka value signifies a stronger acid. In this problem, knowing Ka for HA allows us to relate the acid's dissociation to the equilibrium constant for the reaction with base B, enabling the calculation of K using the relationship between Ka and K.
Recommended video:
Guided course
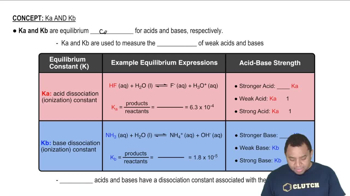
Characteristics of Ka and Kb
Related Practice
Textbook Question
Textbook Question
The acid–base indicator bromcresol green is a weak acid. The yellow acid and blue base forms of the indicator are present in equal concentrations in a solution when the pH is 4.68. What is the pKa for bromcresol green?
Textbook Question
Two buffers are prepared by adding an equal number of moles of formic acid (HCOOH) and sodium formate (HCOONa) to enough water to make 1.00 L of solution. Buffer A is prepared using 1.00 mol each of formic acid and sodium formate. Buffer B is prepared by using 0.010 mol of each. (b) Which buffer will have the greater buffer capacity?
1
views
