The acid–base indicator bromcresol green is a weak acid. The yellow acid and blue base forms of the indicator are present in equal concentrations in a solution when the pH is 4.68. What is the pKa for bromcresol green?
Ch.17 - Additional Aspects of Aqueous Equilibria

Brown14th EditionChemistry: The Central ScienceISBN: 9780134414232Not the one you use?Change textbook
Chapter 17, Problem 88
Two buffers are prepared by adding an equal number of moles of formic acid (HCOOH) and sodium formate (HCOONa) to enough water to make 1.00 L of solution. Buffer A is prepared using 1.00 mol each of formic acid and sodium formate. Buffer B is prepared by using 0.010 mol of each. (a) Calculate the pH of each buffer. (b) Calculate the change in pH for each buffer upon the addition of 1.0 mL of 1.00 M HCl. (c) Calculate the change in pH for each buffer upon the addition of 10 mL of 1.00 M HCl.
 Verified step by step guidance
Verified step by step guidance1
Step 1: Understand the buffer system. A buffer solution consists of a weak acid and its conjugate base. In this case, formic acid (HCOOH) is the weak acid, and sodium formate (HCOONa) is the conjugate base. The pH of a buffer can be calculated using the Henderson-Hasselbalch equation: \( \text{pH} = \text{pK}_a + \log \left( \frac{[\text{A}^-]}{[\text{HA}]} \right) \), where \([\text{A}^-]\) is the concentration of the conjugate base and \([\text{HA}]\) is the concentration of the weak acid.
Step 2: Calculate the pH of Buffer A. For Buffer A, both the formic acid and sodium formate are present at 1.00 M concentration. The \( \text{pK}_a \) of formic acid is approximately 3.75. Substitute these values into the Henderson-Hasselbalch equation to find the pH.
Step 3: Calculate the pH of Buffer B. For Buffer B, both the formic acid and sodium formate are present at 0.010 M concentration. Use the same \( \text{pK}_a \) value and substitute these concentrations into the Henderson-Hasselbalch equation to find the pH.
Step 4: Determine the change in pH for each buffer upon the addition of 1.0 mL of 1.00 M HCl. First, calculate the moles of HCl added. Then, determine how the addition of HCl affects the concentrations of formic acid and sodium formate in each buffer. Use the Henderson-Hasselbalch equation again to find the new pH and calculate the change in pH.
Step 5: Determine the change in pH for each buffer upon the addition of 10 mL of 1.00 M HCl. Repeat the process from Step 4, but with the new volume of HCl. Calculate the moles of HCl added, adjust the concentrations of formic acid and sodium formate, and use the Henderson-Hasselbalch equation to find the new pH and the change in pH.
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Buffer Solutions
Buffer solutions are mixtures that resist changes in pH when small amounts of acid or base are added. They typically consist of a weak acid and its conjugate base, or a weak base and its conjugate acid. In this question, formic acid and sodium formate act as the weak acid and its conjugate base, respectively, allowing the solution to maintain a stable pH despite the addition of HCl.
Recommended video:
Guided course
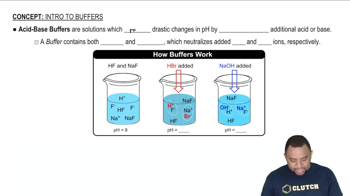
Buffer Solutions
Henderson-Hasselbalch Equation
The Henderson-Hasselbalch equation is a mathematical formula used to calculate the pH of a buffer solution. It is expressed as pH = pKa + log([A-]/[HA]), where pKa is the negative logarithm of the acid dissociation constant, [A-] is the concentration of the conjugate base, and [HA] is the concentration of the weak acid. This equation is essential for determining the pH of the buffers in the question.
Recommended video:
Guided course
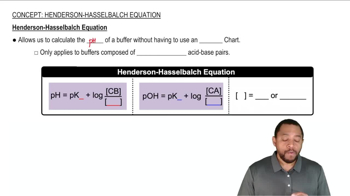
Henderson-Hasselbalch Equation
pH Change upon Acid Addition
When an acid is added to a buffer solution, the pH change depends on the buffer's capacity, which is influenced by the concentrations of the weak acid and its conjugate base. The smaller the concentrations, the more significant the pH change will be upon the addition of a strong acid like HCl. This concept is crucial for analyzing how Buffer A and Buffer B respond to the addition of HCl in the question.
Recommended video:
Guided course

Additional pH and pOH Calculations
Related Practice
Textbook Question
Textbook Question
Two buffers are prepared by adding an equal number of moles of formic acid (HCOOH) and sodium formate (HCOONa) to enough water to make 1.00 L of solution. Buffer A is prepared using 1.00 mol each of formic acid and sodium formate. Buffer B is prepared by using 0.010 mol of each. (b) Which buffer will have the greater buffer capacity?
1
views
Textbook Question
A sample of 0.2140 g of an unknown monoprotic acid was dissolved in 25.0 mL of water and titrated with 0.0950 M NaOH. The acid required 30.0 mL of base to reach the equivalence point. (a) What is the molar mass of the acid?
