Textbook Question
Suggest how the cations in each of the following solution mixtures can be separated: (c) Pb2 + and Al3 +.
1
views

 Verified step by step guidance
Verified step by step guidance
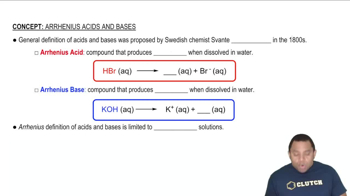
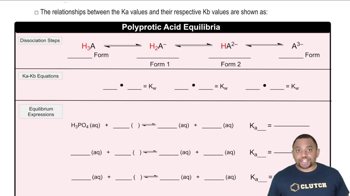
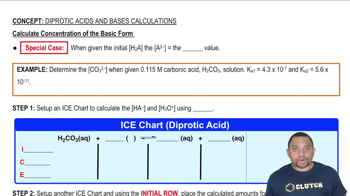
Suggest how the cations in each of the following solution mixtures can be separated: (c) Pb2 + and Al3 +.
(b) What is the most significant difference between the sulfides precipitated in group 2 and those precipitated in group 3?
Derive an equation similar to the Henderson–Hasselbalch equation relating the pOH of a buffer to the pKb of its base component.
The acid–base indicator bromcresol green is a weak acid. The yellow acid and blue base forms of the indicator are present in equal concentrations in a solution when the pH is 4.68. What is the pKa for bromcresol green?