Textbook Question
(b) If the temperature is raised by 100 K, does the equilibrium constant for this reaction increase or decrease?

 Verified step by step guidance
Verified step by step guidance

(b) If the temperature is raised by 100 K, does the equilibrium constant for this reaction increase or decrease?
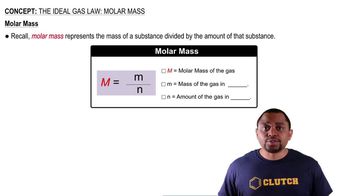
When 2.00 mol of SO2Cl2 is placed in a 2.00-L flask at 303 K, 56% of the SO2Cl2 decomposes to SO2 and Cl2: SO2Cl2(g) ⇌ SO2(g) + Cl2(g) (a) Calculate Kc for this reaction at this temperature.
When 2.00 mol of SO2Cl2 is placed in a 2.00-L flask at 303 K, 56% of the SO2Cl2 decomposes to SO2 and Cl2: SO2Cl2(g) ⇌ SO2(g) + Cl2(g) (c) According to Le Châtelier's principle, would the percent of SO2Cl2 that decomposes increase, decrease or stay the same if the mixture were transferred to a 15.00-L vessel?