Textbook Question
(a) Is the dissociation of fluorine molecules into atomic fluorine, F2(𝑔) ⇌ 2 F(𝑔), an exothermic or endothermic process?

 Verified step by step guidance
Verified step by step guidance
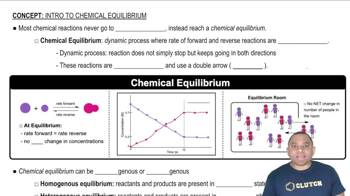
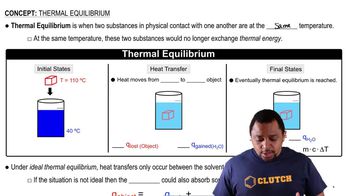
(a) Is the dissociation of fluorine molecules into atomic fluorine, F2(𝑔) ⇌ 2 F(𝑔), an exothermic or endothermic process?
(b) If the temperature is raised by 100 K, does the equilibrium constant for this reaction increase or decrease?
When 2.00 mol of SO2Cl2 is placed in a 2.00-L flask at 303 K, 56% of the SO2Cl2 decomposes to SO2 and Cl2: SO2Cl2(g) ⇌ SO2(g) + Cl2(g) (a) Calculate Kc for this reaction at this temperature.