Textbook Question
When 2.00 mol of SO2Cl2 is placed in a 2.00-L flask at 303 K, 56% of the SO2Cl2 decomposes to SO2 and Cl2: SO2Cl2(g) ⇌ SO2(g) + Cl2(g) (a) Calculate Kc for this reaction at this temperature.
1
views

 Verified step by step guidance
Verified step by step guidance

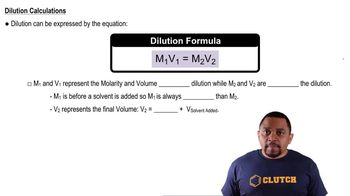
When 2.00 mol of SO2Cl2 is placed in a 2.00-L flask at 303 K, 56% of the SO2Cl2 decomposes to SO2 and Cl2: SO2Cl2(g) ⇌ SO2(g) + Cl2(g) (a) Calculate Kc for this reaction at this temperature.
When 2.00 mol of SO2Cl2 is placed in a 2.00-L flask at 303 K, 56% of the SO2Cl2 decomposes to SO2 and Cl2: SO2Cl2(g) ⇌ SO2(g) + Cl2(g) (c) According to Le Châtelier's principle, would the percent of SO2Cl2 that decomposes increase, decrease or stay the same if the mixture were transferred to a 15.00-L vessel?