 Back
Back- Besides the cubic unit cell, which other unit cell(s) have edge lengths that are all equal to each other? (a) Orthorhombic, (b) hexagonal, (c) rhombohedral, (d) triclinic, (e) both rhombohedral and triclinic.
Problem 26
- What is the minimum number of atoms that could be contained in the unit cell of an element with a body-centered cubic lattice? (a) 1, (b) 2, (c) 3, (d) 4, (e) 5.
Problem 27
Problem 28
What is the minimum number of atoms that could be contained in the unit cell of an element with a face-centered cubic lattice? (a) 1, (b) 2, (c) 3, (d) 4, (e) 5.
Problem 29b
The unit cell of nickel arsenide is shown here. (b) What is the empirical formula?
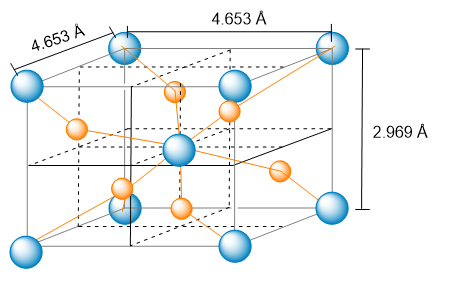
Problem 30a
The unit cell of a compound containing potassium, aluminum, and fluorine is shown here. (a) What type of lattice does this crystal possess (all three lattice vectors are mutually perpendicular)?

- The densities of the elements K, Ca, Sc, and Ti are 0.86, 1.5, 3.2, and 4.5 g/cm³, respectively. One of these elements crystallizes in a body-centered cubic structure; the other three crystallize in a face-centered cubic structure. Which one crystallizes in the body-centered cubic structure? Justify your answer.
Problem 31
- For each of these solids, state whether you would expect it to possess metallic properties: (a) TiCl4 (b) NiCo alloy (c) W (d) Ge (e) ScN
Problem 32
Problem 33a
Consider the unit cells shown here for three different structures that are commonly observed for metallic elements. (a) Which structure(s) corresponds to the densest packing of atoms?

Problem 33b
Consider the unit cells shown here for three different structures that are commonly observed for metallic elements. (b) Which structure(s) corresponds to the least dense packing of atoms?

Problem 34
Sodium metal (atomic weight 22.99 g/mol) adopts a body-centered cubic structure with a density of 0.97 g/cm3. (a) Use this information and Avogadro’s number (NA = 6.022 × 1023/mol) to estimate the atomic radius of sodium. (b) If sodium didn't react so vigorously, it could float on water. Use the answer from part (a) to estimate the density of Na if its structure were that of a cubic close-packed metal. Would it still float on water?
- Iridium crystallizes in a face-centered cubic unit cell that has an edge length of 3.833 Å. (a) Calculate the atomic radius of an iridium atom. (b) Calculate the density of iridium metal.
Problem 35
- Calcium crystallizes in a body-centered cubic structure at 467°C. (b) How many nearest neighbors does each Ca atom possess? (c) Estimate the length of the unit cell edge, a, from the atomic radius of calcium (1.97 Å). (d) Estimate the density of Ca metal at this temperature.
Problem 36
Problem 36a
Calcium crystallizes in a body-centered cubic structure at 467°C. (a) How many Ca atoms are contained in each unit cell?
Problem 36b,c,d
Calcium crystallizes in a body-centered cubic structure at 467°C. (b) How many nearest neighbors does each Ca atom possess? (c) Estimate the length of the unit cell edge, a, from the atomic radius of calcium (1.97 Å). (d) Estimate the density of Ca metal at this temperature.
- Calcium crystallizes in a face-centered cubic unit cell at room temperature that has an edge length of 5.588 Å. (b) Calculate the density of Ca metal at this temperature.
Problem 37
- Calculate the volume in ų of a face-centered cubic unit cell if it is composed of atoms with an atomic radius of 1.82 Å.
Problem 38
- Aluminum metal crystallizes in a face-centered cubic unit cell. (a) How many aluminum atoms are in a unit cell? (b) Estimate the length of the unit cell edge, a, from the atomic radius of aluminum (1.43 Å). (c) Calculate the density of aluminum metal.
Problem 39
Problem 40
An element crystallizes in a face-centered cubic lattice. The edge of the unit cell is 4.078 Å, and the density of the crystal is 19.30 g>cm3. Calculate the atomic weight of the element and identify the element.
Problem 41
Which of these statements about alloys and intermetallic compounds is false? (a) Bronze is an example of an alloy. (b) 'Alloy' is just another word for 'a chemical compound of fixed composition that is made of two or more metals.' (c) Intermetallics are compounds of two or more metals that have a definite composition and are not considered alloys. (d) If you mix two metals together and, at the atomic level, they separate into two or more different compositional phases, you have created a heterogeneous alloy. (e) Alloys can be formed even if the atoms that comprise them are rather different in size.
Problem 42a,c
Determine if each statement is true or false: (a) Substitutional alloys are solid solutions, but interstitial alloys are heterogenous alloys. (c) The atomic radii of the atoms in a substitutional alloy are similar to each other, but in an interstitial alloy, the interstitial atoms are a lot smaller than the host lattice atoms.
Problem 42b
Determine if each statement is true or false: (b) Substitutional alloys have 'solute' atoms that replace 'solvent' atoms in a lattice, but interstitial alloys have 'solute' atoms that are in between the 'solvent' atoms in a lattice.
Problem 43
For each of the following alloy compositions, indicate whether you would expect it to be a substitutional alloy, an interstitial alloy, or an intermetallic compound: (a) Fe0.97Si0.03 (b) Fe0.60Ni0.40 (c) SmCo5.
- For each of the following alloy compositions, indicate whether you would expect it to be a substitutional alloy, an interstitial alloy, or an intermetallic compound: (a) Cu₀.₆₆Zn₀.₃₄ (b) Ag₃Sn (c) Ti₀.₉₉O₀.₀₁.
Problem 44
- Indicate whether each statement is true or false: (a) Substitutional alloys tend to be more ductile than interstitial alloys. (b) Interstitial alloys tend to form between elements with similar ionic radii.
Problem 45
Problem 45c
Indicate whether each statement is true or false: (c) Nonmetallic elements are never found in alloys.
- Indicate whether each statement is true or false: (a) Intermetallic compounds have a fixed composition. (b) Copper is the majority component in both brass and bronze. (c) In stainless steel, the chromium atoms occupy interstitial positions.
Problem 46
- Which element or elements are alloyed with gold to make the following types of 'colored gold' used in the jewelry industry? For each type, also indicate what type of alloy is formed: (c) green gold.
Problem 47
Problem 48
An increase in temperature causes most metals to undergo thermal expansion, which means the volume of the metal increases upon heating. How does thermal expansion affect the unit cell length? What is the effect of an increase in temperature on the density of a metal?
Problem 49a,c,d
State whether each sentence is true or false: (a) Metals have high electrical conductivities because the electrons in the metal are delocalized. (c) Metals have large thermal conductivities because they expand when heated. (d) Metals have small thermal conductivities because the delocalized electrons cannot easily transfer the kinetic energy imparted to the metal from heat.
Problem 49b
State whether each sentence is true or false: (b) Metals have high electrical conductivities because they are denser than other solids.
