The unit cell of nickel arsenide is shown here. (b) What is the empirical formula?

For each of these solids, state whether you would expect it to possess metallic properties: (a) TiCl4 (b) NiCo alloy (c) W (d) Ge (e) ScN
 Verified step by step guidance
Verified step by step guidanceKey Concepts
Metallic Bonding
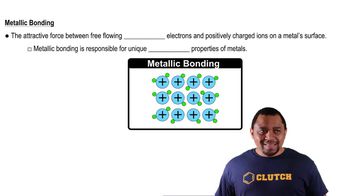
Alloys
Semiconductors

The unit cell of a compound containing potassium, aluminum, and fluorine is shown here. (a) What type of lattice does this crystal possess (all three lattice vectors are mutually perpendicular)?
Consider the unit cells shown here for three different structures that are commonly observed for metallic elements. (a) Which structure(s) corresponds to the densest packing of atoms?
Consider the unit cells shown here for three different structures that are commonly observed for metallic elements. (b) Which structure(s) corresponds to the least dense packing of atoms?
Sodium metal (atomic weight 22.99 g/mol) adopts a body-centered cubic structure with a density of 0.97 g/cm3. (a) Use this information and Avogadro’s number (NA = 6.022 × 1023/mol) to estimate the atomic radius of sodium. (b) If sodium didn't react so vigorously, it could float on water. Use the answer from part (a) to estimate the density of Na if its structure were that of a cubic close-packed metal. Would it still float on water?
