What is the minimum number of atoms that could be contained in the unit cell of an element with a face-centered cubic lattice? (a) 1, (b) 2, (c) 3, (d) 4, (e) 5.
Ch.12 - Solids and Modern Materials

Brown14th EditionChemistry: The Central ScienceISBN: 9780134414232Not the one you use?Change textbook
Chapter 12, Problem 31
The densities of the elements K, Ca, Sc, and Ti are 0.86, 1.5, 3.2, and 4.5 g/cm³, respectively. One of these elements crystallizes in a body-centered cubic structure; the other three crystallize in a face-centered cubic structure. Which one crystallizes in the body-centered cubic structure? Justify your answer.
 Verified step by step guidance
Verified step by step guidance1
Step 1: Understand the difference between body-centered cubic (BCC) and face-centered cubic (FCC) structures. BCC has atoms at each corner of a cube and one atom in the center, while FCC has atoms at each corner and one atom at the center of each face.
Step 2: Recall that the density of a crystalline structure is related to the number of atoms per unit cell, the atomic mass, and the volume of the unit cell. BCC structures typically have lower packing efficiency compared to FCC structures.
Step 3: Calculate the packing efficiency for both BCC and FCC structures. BCC has a packing efficiency of about 68%, while FCC has a packing efficiency of about 74%.
Step 4: Compare the given densities of the elements (K: 0.86 g/cm³, Ca: 1.5 g/cm³, Sc: 3.2 g/cm³, Ti: 4.5 g/cm³) with typical densities for BCC and FCC structures. BCC structures generally have lower densities due to their lower packing efficiency.
Step 5: Identify the element with the lowest density, which is potassium (K) with a density of 0.86 g/cm³. This suggests that potassium is the element that crystallizes in a body-centered cubic structure due to its lower density compared to the others.
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Crystal Structures
Crystal structures refer to the orderly arrangement of atoms in a crystalline solid. The two common types are body-centered cubic (BCC) and face-centered cubic (FCC). In BCC, atoms are located at each corner of a cube and one atom at the center, while in FCC, atoms are at each corner and the centers of all the cube faces. Understanding these structures helps in predicting the properties and densities of elements.
Recommended video:
Guided course
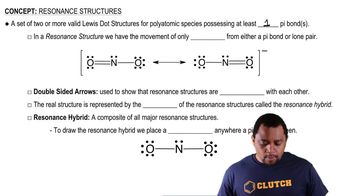
Resonance Structures
Density and Atomic Arrangement
Density is defined as mass per unit volume and is influenced by the arrangement of atoms in a crystal lattice. Elements with a BCC structure typically have lower densities compared to those with FCC due to the less efficient packing of atoms. By analyzing the given densities, one can infer which element is likely to have a BCC structure based on its relatively lower density.
Recommended video:
Guided course
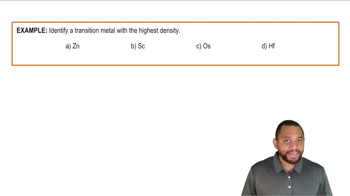
Atomic Radius and Density of Transition Metals Example
Periodic Trends and Element Properties
Periodic trends refer to patterns in the properties of elements as you move across or down the periodic table. Elements in the same group often exhibit similar properties, including density and crystal structure. By considering the position of K, Ca, Sc, and Ti in the periodic table, one can make educated guesses about their crystallization behavior and deduce which element is likely to adopt a BCC structure.
Recommended video:
Guided course
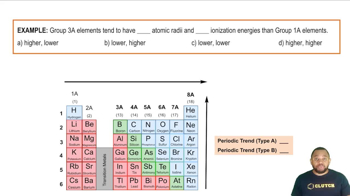
Main Group Elements: Periodic Trends Example
Related Practice
Textbook Question
Textbook Question
The unit cell of nickel arsenide is shown here. (b) What is the empirical formula?
Textbook Question
The unit cell of a compound containing potassium, aluminum, and fluorine is shown here. (a) What type of lattice does this crystal possess (all three lattice vectors are mutually perpendicular)?
Textbook Question
Consider the unit cells shown here for three different structures that are commonly observed for metallic elements. (a) Which structure(s) corresponds to the densest packing of atoms?
Textbook Question
Consider the unit cells shown here for three different structures that are commonly observed for metallic elements. (b) Which structure(s) corresponds to the least dense packing of atoms?
