Based on their composition and structure, list CH2Cl2, CH3CH2CH3, and CH3CH2OH in order of (a) increasing intermolecular forces (c) increasing surface tension
Ch.11 - Liquids and Intermolecular Forces

Brown14th EditionChemistry: The Central ScienceISBN: 9780134414232Not the one you use?Change textbook
Chapter 11, Problem 37
The boiling points, surface tensions, and viscosities of water and several alcohols are as shown below: (b) How do you explain the fact that propanol and ethylene glycol have similar molecular weights (60 versus 62 amu), yet the viscosity of ethylene glycol is more than 10 times larger than propa- nol?
 Verified step by step guidance
Verified step by step guidance1
Identify the molecular structures of propanol and ethylene glycol. Propanol is C3H7OH, and ethylene glycol is C2H4(OH)2.
Recognize that both molecules have similar molecular weights, but ethylene glycol has two hydroxyl (OH) groups, while propanol has only one.
Understand that the presence of more hydroxyl groups in ethylene glycol leads to stronger hydrogen bonding compared to propanol.
Explain that stronger hydrogen bonding in ethylene glycol results in higher intermolecular forces, which increases its viscosity.
Conclude that the increased viscosity of ethylene glycol compared to propanol is due to its ability to form more extensive hydrogen bonding networks.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
59sWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
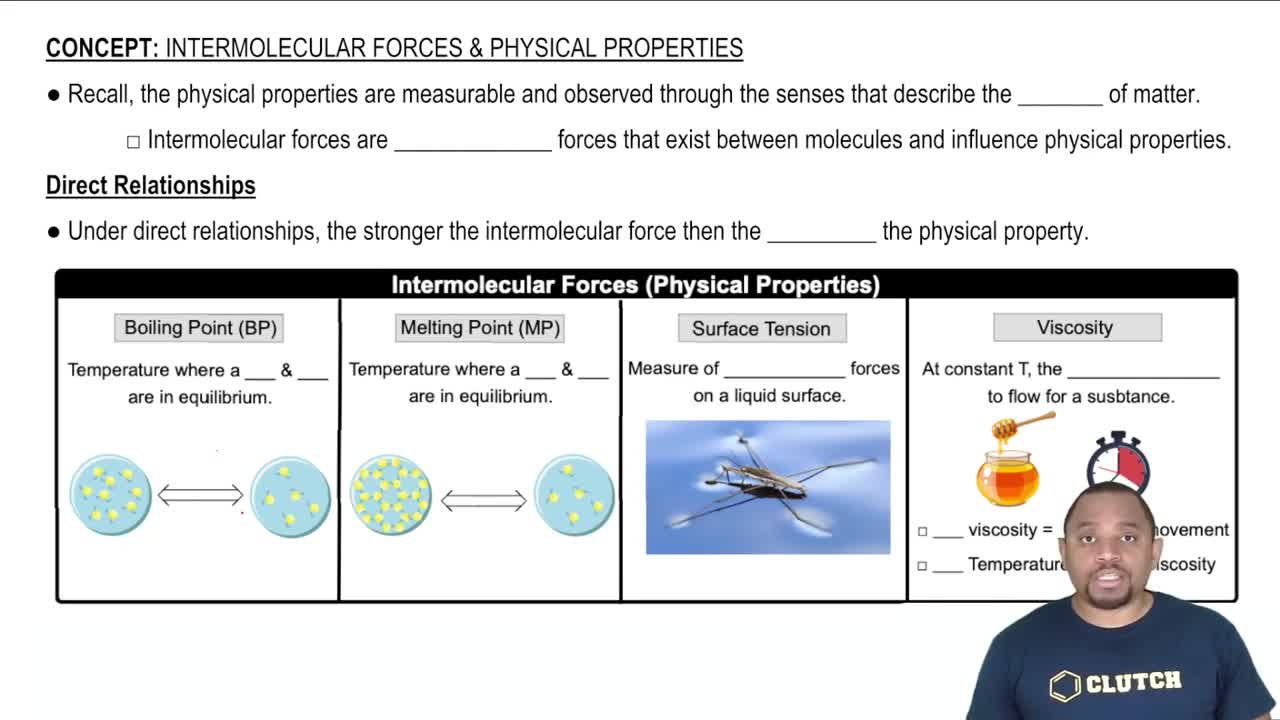
Intermolecular Forces
Intermolecular forces are the attractive forces between molecules that influence physical properties such as boiling point, viscosity, and surface tension. Ethylene glycol has stronger hydrogen bonding due to its two hydroxyl (-OH) groups, leading to higher viscosity compared to propanol, which has only one -OH group. This difference in hydrogen bonding significantly affects how the molecules interact with each other.
Recommended video:
Guided course

Intermolecular vs Intramolecular Forces
Viscosity
Viscosity is a measure of a fluid's resistance to flow, which is influenced by the size and shape of molecules, as well as the strength of intermolecular forces. Higher viscosity indicates that a liquid flows less easily. In the case of ethylene glycol, its ability to form extensive hydrogen bonds results in a more structured network, increasing its viscosity compared to propanol.
Recommended video:
Guided course
Intermolecular Forces and Properties
Molecular Weight vs. Physical Properties
While molecular weight can provide some insight into the properties of a substance, it does not solely determine characteristics like viscosity. Ethylene glycol and propanol have similar molecular weights, but their differing structures and the number of functional groups lead to significant differences in their physical properties. This highlights the importance of molecular structure in understanding the behavior of substances.
Recommended video:
Guided course
Physical Properties
Related Practice
Textbook Question
Textbook Question
Liquids can interact with flat surfaces just as they can with capillary tubes; the cohesive forces within the liquid can be stronger or weaker than the adhesive forces between liquid and surface:
(b) Which of these diagrams, i or ii, rep- resents what happens when water is on a nonpolar surface?
Textbook Question
Name the phase transition in each of the following situations and indicate whether it is exothermic or endothermic: (c) Rubbing alcohol in an open container slowly disappears.
Textbook Question
Name the phase transition in each of the following situations and indicate whether it is exothermic or endothermic: (d) Molten lava from a volcano turns into solid rock.
