The generic structural formula for a 1-alkyl-3-methylimid- azolium cation is where R is a -CH2(CH2)nCH3 alkyl group. The melting points of the salts that form between 1-alkyl-3-methylimidazolium cation and the PF6- anion are as follows: R = CH2CH3 (m.p. = 60 °C), R = CH2CH2CH3 (m.p. = 40 °C), r = CH2CH2CH2CH3 (m.p. = 10 °C), and R = CH2CH2CH2CH2CH2CH3 (m.p. = -61 °C). Why does the melting point decrease as the length of alkyl group increases?
Ch.11 - Liquids and Intermolecular Forces

Brown14th EditionChemistry: The Central ScienceISBN: 9780134414232Not the one you use?Change textbook
Chapter 11, Problem 34a,c
Based on their composition and structure, list CH2Cl2, CH3CH2CH3, and CH3CH2OH in order of (a) increasing intermolecular forces (c) increasing surface tension
 Verified step by step guidance
Verified step by step guidance1
Identify the molecular structure of each compound: CH\(_2\)Cl\(_2\) (dichloromethane), CH\(_3\)CH\(_2\)CH\(_3\) (propane), and CH\(_3\)CH\(_2\)OH (ethanol).
Consider the types of intermolecular forces present in each compound. CH\(_2\)Cl\(_2\) has dipole-dipole interactions due to its polar nature, CH\(_3\)CH\(_2\)CH\(_3\) has only London dispersion forces as it is nonpolar, and CH\(_3\)CH\(_2\)OH has hydrogen bonding due to the presence of an -OH group.
Recall that surface tension is influenced by the strength of intermolecular forces: stronger intermolecular forces result in higher surface tension.
Compare the strength of intermolecular forces: hydrogen bonding (strongest) > dipole-dipole interactions > London dispersion forces (weakest).
Arrange the compounds in order of increasing surface tension based on the strength of their intermolecular forces: CH\(_3\)CH\(_2\)CH\(_3\) (weakest) < CH\(_2\)Cl\(_2\) < CH\(_3\)CH\(_2\)OH (strongest).

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
1mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Surface Tension
Surface tension is a physical property of liquids that describes the elastic-like force at the surface, caused by the cohesive forces between liquid molecules. It is influenced by intermolecular forces, such as hydrogen bonding and van der Waals forces. Liquids with stronger intermolecular forces typically exhibit higher surface tension, as the molecules at the surface are pulled inward more strongly.
Recommended video:
Guided course
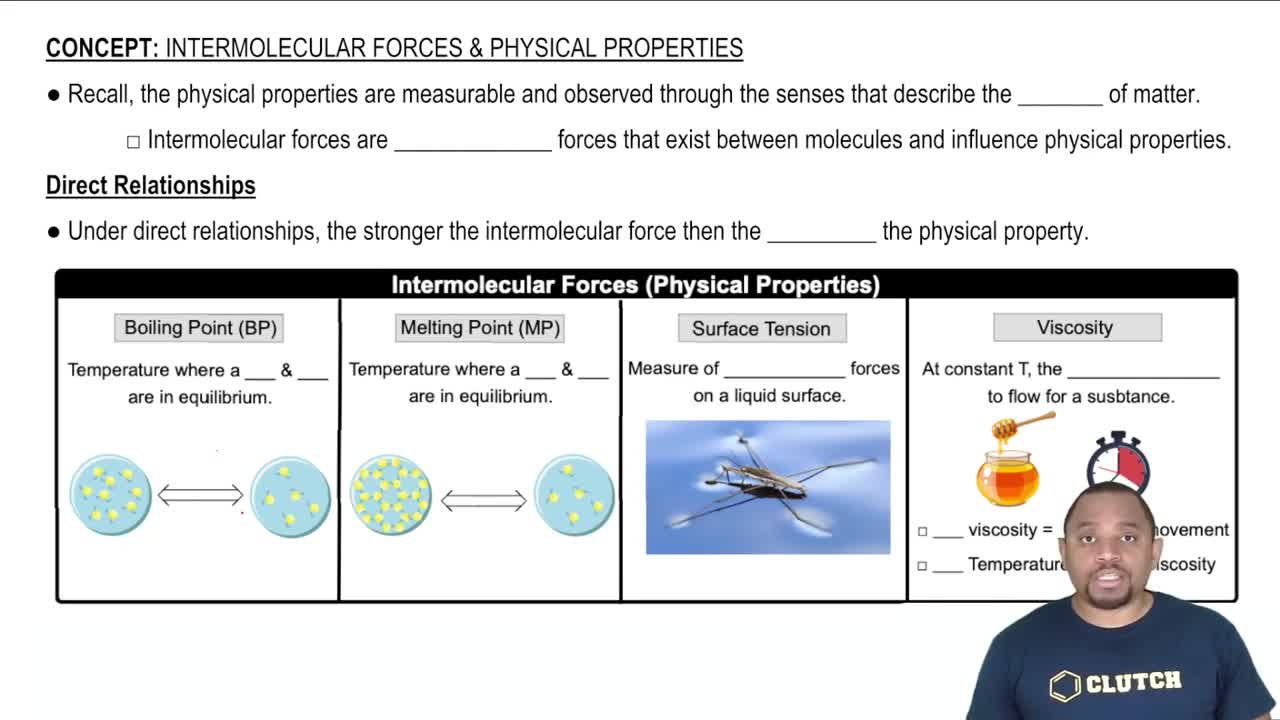
Intermolecular Forces and Properties
Intermolecular Forces
Intermolecular forces are the forces of attraction or repulsion between molecules. They include hydrogen bonds, dipole-dipole interactions, and London dispersion forces. The type and strength of these forces significantly affect the physical properties of substances, including boiling point, melting point, and surface tension. For example, molecules with hydrogen bonding, like alcohols, generally have higher surface tension than non-polar molecules.
Recommended video:
Guided course
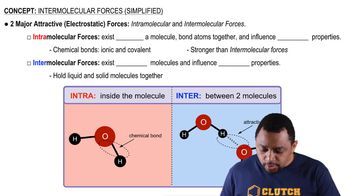
Intermolecular vs Intramolecular Forces
Molecular Structure and Polarity
The molecular structure and polarity of a compound play a crucial role in determining its physical properties. Polar molecules, which have a significant difference in electronegativity between atoms, tend to have stronger intermolecular forces compared to non-polar molecules. In the context of the given compounds, the presence of polar functional groups, such as hydroxyl (-OH) in CH3CH2OH, increases surface tension due to enhanced hydrogen bonding compared to non-polar hydrocarbons.
Recommended video:
Guided course
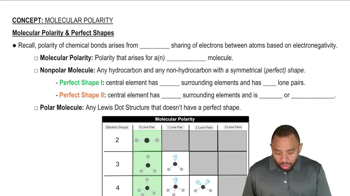
Molecular Polarity
Related Practice
Textbook Question
Textbook Question
(b) What is the relationship between viscosity and temperature?
Textbook Question
(c) Why do substances with high surface tension also tend to have high viscosities?
Textbook Question
Liquids can interact with flat surfaces just as they can with capillary tubes; the cohesive forces within the liquid can be stronger or weaker than the adhesive forces between liquid and surface:
(b) Which of these diagrams, i or ii, rep- resents what happens when water is on a nonpolar surface?
Textbook Question
The boiling points, surface tensions, and viscosities of water and several alcohols are as shown below: (b) How do you explain the fact that propanol and ethylene glycol have similar molecular weights (60 versus 62 amu), yet the viscosity of ethylene glycol is more than 10 times larger than propa- nol?
