Multiple Choice
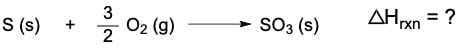
Given the following reactions:2S (s) + 3O2 (g) → 2SO3 (g) ΔH = -790 kJS (s) + O2 (g) → SO2 (g) ΔH = -297 kJWhat is the enthalpy change (ΔH) for the reaction in which sulfur dioxide is oxidized to sulfur trioxide: 2SO2 (g) + O2 (g) → 2SO3 (g)?