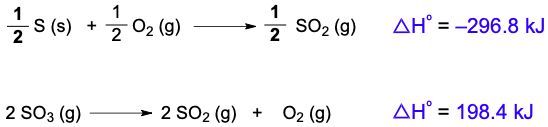
Multiple Choice
Using Hess's Law, calculate the standard Gibbs free energy change (ΔG°_rxn) for the reaction: ClO(g) + O3(g) → Cl(g) + 2 O2(g), given the following reactions and their ΔG°_rxn values: 1) 2 O3(g) → 3 O2(g), ΔG°_rxn = +489.6 kJ; 2) Cl(g) + O3(g) → ClO(g) + O2(g), ΔG°_rxn = -101.3 kJ.