Which of the following complexes are paramagnetic?
(a) [Mn(CN)6]3-
(b) [Zn(NH3)4]2+ (tetrahedral)
(c) [Fe(CN)6]4-
(d) [FeF6]4-

 McMurry 8th Edition
McMurry 8th Edition Ch.21 - Transition Elements and Coordination Chemistry
Ch.21 - Transition Elements and Coordination Chemistry Problem 21.120
Problem 21.120 Verified step by step guidance
Verified step by step guidance

Which of the following complexes are paramagnetic?
(a) [Mn(CN)6]3-
(b) [Zn(NH3)4]2+ (tetrahedral)
(c) [Fe(CN)6]4-
(d) [FeF6]4-
Draw a crystal field energy-level diagram, assign the electrons to orbitals, and predict the number of unpaired electrons for each of the following.
(a) [Cu(en)3]2+
(b) [FeF6]2-
(c) [Co(en)3]3+ (low spin)
What is the systematic name for each of the following coordination compounds?
(c) [Co(NH3)4Br2]Br
(d) Cu(gly)2
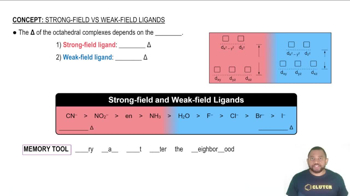
The amount of paramagnetism for a first-series transition metal complex is related approximately to its spin-only magnetic moment. The spin-only value of the magnetic moment in units of Bohr magnetons (BM) is given by sqrt(n(n + 2)), where n is the number of unpaired electrons. Calculate the spin-only value of the magnetic moment for the 2+ ions of the first-series transition metals (except Sc) in octahedral complexes with (a) weak-field ligands and (b) strong-field ligands. For which electron configurations can the magnetic moment distinguish between high-spin and low-spin electron configurations?
What hybrid orbitals are used by the metal ion and how many unpaired electrons are present the complex ion [VCl4]- with tetrahedral geometry?
(a) sp3; 2 unpaired electrons
(b) sp3; 3 unpaired electrons
(c) sp3d2; 3 unpaired electrons
(d) sp3d2; 4 unpaired electrons
Draw the structure of all isomers of the octahedral complex [NbX2Cl4]- (X- = NCS-), and identify those that are linkage isomers.