Predict the number of unpaired electrons for each of the following.
(c) Zn2+
(d) Cr3+

 McMurry 8th Edition
McMurry 8th Edition Ch.21 - Transition Elements and Coordination Chemistry
Ch.21 - Transition Elements and Coordination Chemistry Problem 21.121
Problem 21.121 Verified step by step guidance
Verified step by step guidance

Predict the number of unpaired electrons for each of the following.
(c) Zn2+
(d) Cr3+
What is the general trend in standard potentials for the oxidation of first-series transition metals from Sc to Zn? What is the reason for the trend?
What is the crystal field energy level diagram for the complex [Fe(NH3)6]3+?
(a)
(b)
(c)
(d)
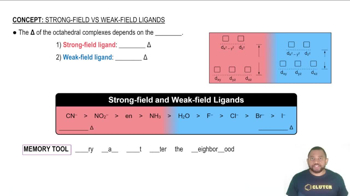
Based on the wavelength of maximum absorption of the cobalt complexes, arrange the following ligands in a spectrochemical series from weakest-field to strongest-field ligand.
(a) Cl- < NCS- < H2O < NH3
(b) Cl- < NCS- < H2O < NH3
(c) H2O < Cl- < NH3 < NCS-
(d) Cl- < H2O < NCS- < NH3
For each of the following complexes, draw a crystal field energy-level diagram, assign the electrons to orbitals, and predict the number of unpaired electrons.
(a) [CrF6]3-
(b) [V(H2O)6]3+
(c) [Fe(CN)6]3-
Which of the following complexes are paramagnetic?
(a) [Mn(CN)6]3-
(b) [Zn(NH3)4]2+ (tetrahedral)
(c) [Fe(CN)6]4-
(d) [FeF6]4-