The [Cr(H2O)6]3+ ion is violet, and [Cr(CN)6]3- is yellow. Explain this difference using crystal field theory. Use the colors to order H2O and CN- in the spectrochemical series.

 McMurry 8th Edition
McMurry 8th Edition Ch.21 - Transition Elements and Coordination Chemistry
Ch.21 - Transition Elements and Coordination Chemistry Problem 21.116
Problem 21.116Although Cl- is a weak-field ligand and CN- is a strong field ligand, [CrCl6]3- and [Cr(CN)6]3- exhibit approximately the same amount of paramagnetism. Explain.
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
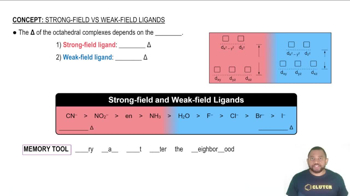
Ligand Field Theory
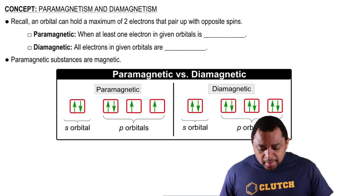
Paramagnetism
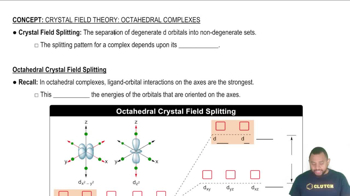
Crystal Field Splitting
Draw the structures of all possible diastereoisomers of an octahedral complex with the formula MA2B2C2. Which of the diastereoisomers, if any, can exist as enantiomers?
Predict the crystal field energy-level diagram for a square pyramidal ML5 complex that has two ligands along the axes but only one ligand along the z axis. Your diagram should be intermediate between those for an octahedral ML6 complex and a square planar ML4 complex.
The Ni2+(aq) cation is green, but Zn2+(aq) is colorless. Explain.
The glycinate anion, gly-= NH2CH2CO2 -, bonds to metal ions through the N atom and one of the O atoms. Using to represent gly-, sketch the structures of the four stereoisomers of Co(gly)3.
What is the oxidation state of the metal in each of the complexes?
a. [Ni(CN)5]3–
b. Ni(CO)4
c. [Co(en)2(H2O)Br]2+
d. [Cu(H2O)2(C2O4)2]2–
e. Co(NH3)3(NO2)3