Textbook Question
You have a sample of gas at 0 C. You wish to increase therms speed by a factor of 3. To what temperature shouldthe gas be heated?

 Verified step by step guidance
Verified step by step guidance


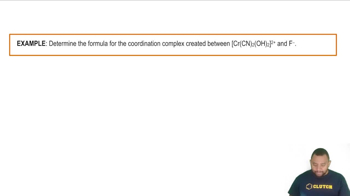
Consider the following gases, all at STP: Ne, SF6, N2, CH4. (d) Which one has the highest total molecular volume relative to the space occupied by the gas?
Consider the following gases, all at STP: Ne, SF6, N2, CH4. (f) Which one would effuse more rapidly than N2?
Consider the following gases, all at STP: Ne, SF6, N2, CH4. (g) Which one would have the largest van der Waals b parameter?