Textbook Question
The density of a gas of unknown molar mass was measured as a function of pressure at 0 C, as in the table that follows. (b) Why is d>P not a constant as a function of pressure?

 Verified step by step guidance
Verified step by step guidance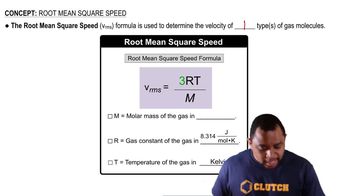

The density of a gas of unknown molar mass was measured as a function of pressure at 0 C, as in the table that follows. (b) Why is d>P not a constant as a function of pressure?
Consider the following gases, all at STP: Ne, SF6, N2, CH4. (a) Which gas is most likely to depart from the assumption of the kinetic-molecular theory that says there are no attractive or repulsive forces between molecules?
Consider the following gases, all at STP: Ne, SF6, N2, CH4. (d) Which one has the highest total molecular volume relative to the space occupied by the gas?
Consider the following gases, all at STP: Ne, SF6, N2, CH4. (f) Which one would effuse more rapidly than N2?