The density of a gas of unknown molar mass was measured as a function of pressure at 0 C, as in the table that follows. (a) Determine a precise molar mass for the gas. [Hint: Graph d>P versus P.]
Ch.10 - Gases

Brown14th EditionChemistry: The Central ScienceISBN: 9780134414232Not the one you use?Change textbook
Chapter 10, Problem 112
You have a sample of gas at 0 C. You wish to increase therms speed by a factor of 3. To what temperature shouldthe gas be heated?
 Verified step by step guidance
Verified step by step guidance1
Identify the initial temperature in Kelvin. Since the given temperature is 0°C, convert it to Kelvin by adding 273.15.
Recognize that the root mean square (rms) speed of a gas is proportional to the square root of its temperature. The formula relating rms speed and temperature is \( v_{rms} \propto \sqrt{T} \).
Set up the proportionality equation for the rms speeds before and after the temperature change. If the initial rms speed is \( v_{rms, initial} \) and the final rms speed is \( v_{rms, final} = 3 \times v_{rms, initial} \), then \( \frac{v_{rms, final}}{v_{rms, initial}} = \frac{\sqrt{T_{final}}}{\sqrt{T_{initial}}} \).
Solve the proportionality equation for \( T_{final} \). Since \( \frac{v_{rms, final}}{v_{rms, initial}} = 3 \), square both sides to remove the square root, resulting in \( \frac{T_{final}}{T_{initial}} = 9 \).
Calculate the final temperature, \( T_{final} \), by multiplying the initial temperature in Kelvin by 9.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
2mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Root Mean Square Speed (rms speed)
The root mean square speed is a measure of the average speed of gas particles in a sample. It is calculated using the formula v_rms = √(3RT/M), where R is the ideal gas constant, T is the absolute temperature in Kelvin, and M is the molar mass of the gas. This concept is crucial for understanding how temperature affects the kinetic energy and speed of gas molecules.
Recommended video:
Guided course
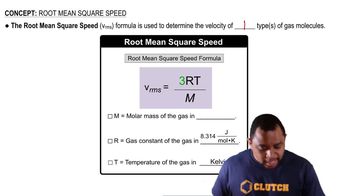
Root Mean Square Speed Formula
Temperature and Kinetic Energy
Temperature is a measure of the average kinetic energy of the particles in a substance. In gases, as temperature increases, the kinetic energy of the molecules also increases, leading to higher speeds. This relationship is fundamental in determining how much the temperature must be raised to achieve a desired increase in rms speed.
Recommended video:
Guided course
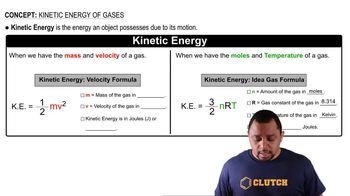
Kinetic Energy Formulas
Ideal Gas Law
The Ideal Gas Law, represented as PV = nRT, relates the pressure (P), volume (V), number of moles (n), gas constant (R), and temperature (T) of an ideal gas. This law provides a framework for understanding the behavior of gases under various conditions and is essential for calculating changes in temperature when manipulating other variables, such as speed.
Recommended video:
Guided course

Ideal Gas Law Formula
Related Practice
Textbook Question
Textbook Question
The density of a gas of unknown molar mass was measured as a function of pressure at 0 C, as in the table that follows. (b) Why is d>P not a constant as a function of pressure?
Textbook Question
Consider the following gases, all at STP: Ne, SF6, N2, CH4. (a) Which gas is most likely to depart from the assumption of the kinetic-molecular theory that says there are no attractive or repulsive forces between molecules?
Textbook Question
Consider the following gases, all at STP: Ne, SF6, N2, CH4. (d) Which one has the highest total molecular volume relative to the space occupied by the gas?
