Textbook Question
You have a sample of gas at 0 C. You wish to increase therms speed by a factor of 3. To what temperature shouldthe gas be heated?

 Verified step by step guidance
Verified step by step guidance

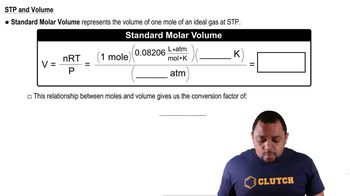

Consider the following gases, all at STP: Ne, SF6, N2, CH4. (a) Which gas is most likely to depart from the assumption of the kinetic-molecular theory that says there are no attractive or repulsive forces between molecules?
Consider the following gases, all at STP: Ne, SF6, N2, CH4. (f) Which one would effuse more rapidly than N2?
Consider the following gases, all at STP: Ne, SF6, N2, CH4. (g) Which one would have the largest van der Waals b parameter?