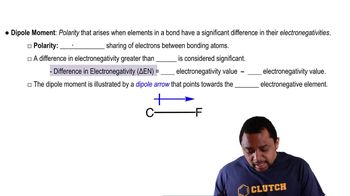
Tell how many diastereoisomers are possible for each of the following complexes, and draw their structures.
(c) [Cu(H2O)4Cl2]+
(d) Ru(NH3)3I3

 McMurry 8th Edition
McMurry 8th Edition Ch.21 - Transition Elements and Coordination Chemistry
Ch.21 - Transition Elements and Coordination Chemistry Problem 21.140b
Problem 21.140b Verified step by step guidance
Verified step by step guidance
Tell how many diastereoisomers are possible for each of the following complexes, and draw their structures.
(c) [Cu(H2O)4Cl2]+
(d) Ru(NH3)3I3
Assign a systematic name to each of the following ions.
(c) [Fe(H2O)5NCS]2+
(d) [Cr(NH3)2(C2O4)2]-
Predict the number of unpaired electrons for each of the following.
(c) Mn3+
(d) Cr2+
For each of the following complexes, draw a crystal field energy-level diagram, assign the electrons to orbitals, and predict the number of unpaired electrons.
(b) [MnCl4]2- (tetrahedral)
For each of the following complexes, describe the bonding using valence bond theory. Include orbital diagrams for the free metal ion and the metal ion in the complex. Indicate which hybrid orbitals the metal ion uses for bonding, and specify the number of unpaired electrons.
(b) [Ag(NH3)2]+
What is the systematic name for each of the following ions?
(c) [Co(CO3)3]3-
(d) [Pt(en)2(SCN)2]2+