Textbook Question
Two systems, each composed of two particles represented by circles, have 20 J of total energy. Which system, A or B, has the greater entropy? Why?

 Verified step by step guidance
Verified step by step guidance
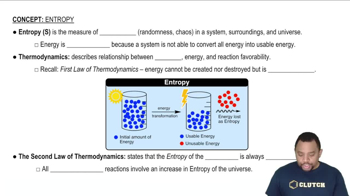
Two systems, each composed of two particles represented by circles, have 20 J of total energy. Which system, A or B, has the greater entropy? Why?
Two systems, each composed of three particles represented by circles, have 30 J of total energy. How many energetically equivalent ways can you distribute the particles in each system? Which system has greater entropy?
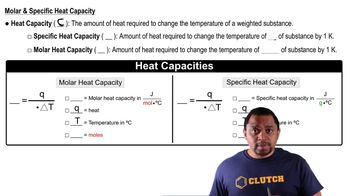
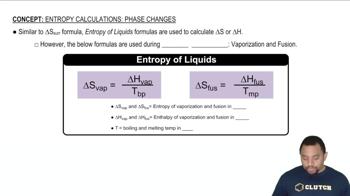
Calculate the change in entropy that occurs in the system when 1.00 mole of isopropyl alcohol (C3H8O) melts at its melting point (-89.5 °C). See Table 11.9 for heats of fusion.
Without doing any calculations, determine the sign of ΔSsys for each chemical reaction. a. 2 KClO3(s) → 2 KCl(s) + 3 O2(g) c. Na(s) + 2 Cl2(g) → NaCl(s) d. N2(g) + 3 H2(g) → 2 NH3(g)