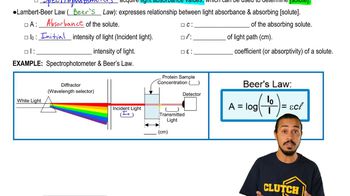
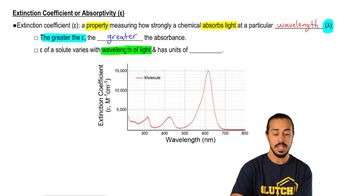
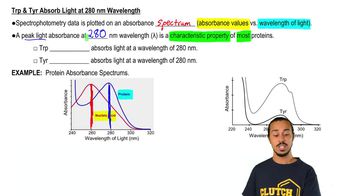
5. Protein Techniques
Spectrophotometry
Practice this topic
- Multiple Choice
What is the relationship between light absorbance (A) & the amount of light transmitted through a sample?
- Multiple Choice
Which of the following options is false for Beer's Law?
- Open Question
A) Suppose myoglobin's molecular weight is 17,800 g/mole and its extinction coefficient at 280 nm wavelength is 15,000 M-1 cm-1. What is the absorbance of a myoglobin solution (concentration = 1 mg/mL) across a 1-cm path?
Hint: Use Beer's law.
a. 0.49
b. 0.73
c. 0.36
d. 0.84
B) What is the percentage of the incident light that is transmitted through this solution?
a. 14%
b. 6%
c. 21%
d. 58%
- Multiple Choice
A protein solution has an absorbance of 0.1 at 280 nm with a path length of 1 cm. If the protein sequence includes 3 Trp residues but no other aromatic residues, what is the concentration of the protein? (Trp ε = 3,400 M-1 cm-1).
- Open Question
An unknown protein has been isolated in your laboratory and determined to have 172 amino acids but does not have tryptophan. You have been asked to determine the possible tyrosine content of this protein. You know from your study of this lesson that there is a relatively easy way to do this. You prepare a pure 50 μM solution of the protein, and you place it in a sample cell with a 1-cm path length, and you measure the absorbance of this sample at 280 nm in a UV-visible spectrophotometer. The absorbance of the solution is 0.398. How many tyrosine residues are there in this protein? (Tyr ε ≈ 1,000 M-1 cm-1 ).