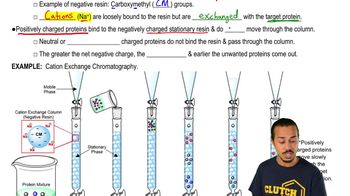
5. Protein Techniques
Ion-Exchange Chromatography
Practice this topic
- Multiple Choice
What is the order of elution of the following proteins from a cation-exchange chromatography column?
Net charges of Proteins: Protein A = +1 Protein B = -2 Protein C = -5 Protein D = +3.
- Multiple Choice
In a cation-exchange column at neutral pH, which peptide would elute last?
- Open Question
Mixtures of amino acids can be analyzed by first separating the mixture into its components through ion exchange chromatography. Certain amino acids placed on a cation-exchange resin containing sulfonate groups (—SO3-) flow down the column slowly because of two factors that influence their movement: (1) ionic attraction between the sulfonate residues on the column and positively charged functional groups on the amino acids, and (2) hydrophobic interactions between amino acid R-groups and the strongly hydrophobic backbone of the polystyrene resin. For each pair of amino acids listed below, circle the amino acid that is eluted first from the cation-exchange column by a buffer at pH 7.
- Open Question
Give the order of elution of the following peptides when using cation-exchange chromatography at pH 7.2.
Peptide #1: A-D-G-H-E. Peptide #2: K-L-M-R-A. Peptide #3: M-D-L-I-V. Peptide #4: I-L-R-P-M.
Order of Elution: _______________, _______________, _______________, _______________
(1 st to elute) (Last to elute)