Back
BackProblem 1a
Two moles of an ideal gas are heated at constant pressure from °C to °C. Draw a -diagram for this process.
Problem 1b
Two moles of an ideal gas are heated at constant pressure from °C to °C. Calculate the work done by the gas.
Problem 2
Six moles of an ideal gas are in a cylinder fitted at one end with a movable piston. The initial temperature of the gas is °C and the pressure is constant. As part of a machine design project, calculate the final temperature of the gas after it has done J of work.
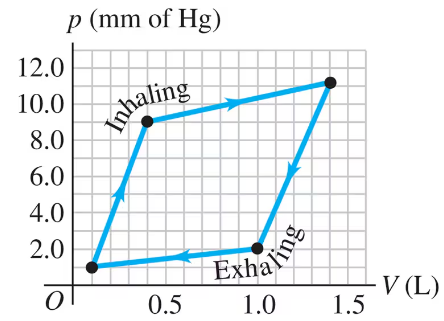
Problem 4a
The graph in Fig. E shows a -diagram of the air in a human lung when a person is inhaling and then exhaling a deep breath. Such graphs, obtained in clinical practice, are normally somewhat curved, but we have modeled one as a set of straight lines of the same general shape. (Important: The pressure shown is the gauge pressure, not the absolute pressure.) How many joules of net work does this person's lung do during one complete breath?
Problem 4b
The graph in Fig. E shows a -diagram of the air in a human lung when a person is inhaling and then exhaling a deep breath. Such graphs, obtained in clinical practice, are normally somewhat curved, but we have modeled one as a set of straight lines of the same general shape. (Important: The pressure shown is the gauge pressure, not the absolute pressure.) The process illustrated here is somewhat different from those we have been studying, because the pressure change is due to changes in the amount of gas in the lung, not to temperature changes. (Think of your own breathing. Your lungs do not expand because they've gotten hot.) If the temperature of the air in the lung remains a reasonable °C, what is the maximum number of moles in this person's lung during a breath?
Problem 6a
A gas undergoes two processes. In the first, the volume remains constant at m3 and the pressure increases from Pa to Pa. The second process is a compression to a volume of m3 at a constant pressure of Pa. In a -diagram, show both processes.
Problem 6b
A gas undergoes two processes. In the first, the volume remains constant at m3 and the pressure increases from Pa to Pa. The second process is a compression to a volume of m3 at a constant pressure of Pa. Find the total work done by the gas during both processes.
Problem 7a
In Fig. a, consider the closed loop . This is a cyclic process in which the initial and final states are the same. Find the total work done by the system in this cyclic process, and show that it is equal to the area enclosed by the loop.

Problem 9a
A gas in a cylinder expands from a volume of m3 to m3 . Heat flows into the gas just rapidly enough to keep the pressure constant at Pa during the expansion. The total heat added is J. Find the work done by the gas.
Problem 10
Five moles of an ideal monatomic gas with an initial temperature of °C expand and, in the process, absorb J of heat and do J of work. What is the final temperature of the gas?
Problem 11a
The process shown in the -diagram in Fig. E involves mol of an ideal gas. What was the lowest temperature the gas reached in this process? Where did it occur?

Problem 12c
A gas in a cylinder is held at a constant pressure of Pa and is cooled and compressed from m3 to m3. The internal energy of the gas decreases by J. Does it matter whether the gas is ideal? Why or why not?
Problem 13c
The -diagram in Fig. E shows a process involving mol of an ideal gas. How much heat had to be added during the process to increase the internal energy of the gas by J?

Problem 14a
When water is boiled at a pressure of atm, the heat of vaporization is J/kg and the boiling point is °C. At this pressure, kg of water has a volume of m3, and kg of steam has a volume of m3. Compute the work done when kg of steam is formed at this temperature.
Problem 14b
When water is boiled at a pressure of atm, the heat of vaporization is J/kg and the boiling point is °C. At this pressure, kg of water has a volume of m3, and kg of steam has a volume of m3. Compute the increase in internal energy of the water.
Problem 16
During an isothermal compression of an ideal gas, J of heat must be removed from the gas to maintain constant temperature. How much work is done by the gas during the process?
Problem 18a
A cylinder contains mol of helium at °C. How much heat is needed to raise the temperature to °C while keeping the volume constant? Draw a -diagram for this process.
Problem 18b
A cylinder contains mol of helium at °C. If instead the pressure of the helium is kept constant, how much heat is needed to raise the temperature from °C to °C? Draw a -diagram for this process.
Problem 18c
A cylinder contains mol of helium at °C. What accounts for the difference between your answers to parts (a) and (b)? In which case is more heat required? What becomes of the additional heat?
(a) How much heat is needed to raise the temperature to °C while keeping the volume constant? Draw a -diagram for this process.
(b) If instead the pressure of the helium is kept constant, how much heat is needed to raise the temperature from °C to °C? Draw a -diagram for this process.
Problem 18d
A cylinder contains mol of helium at °C. If the gas is ideal, what is the change in its internal energy in part (a)? In part (b)? How do the two answers compare? Why?
(a) How much heat is needed to raise the temperature to °C while keeping the volume constant? Draw a -diagram for this process.
(b) If instead the pressure of the helium is kept constant, how much heat is needed to raise the temperature from °C to °C? Draw a -diagram for this process.
Problem 21
Heat flows into a monatomic ideal gas, and the volume increases while the pressure is kept constant. What fraction of the heat energy is used to do the expansion work of the gas?
Problem 23b
An experimenter adds J of heat to mol of an ideal gas to heat it from °C to °C at constant pressure. The gas does J of work during the expansion. Calculate for the gas.
Problem 25c
The temperature of mol of an ideal gas is held constant at °C while its volume is reduced to of its initial volume. The initial pressure of the gas is atm. Does the gas exchange heat with its surroundings? If so, how much? Does the gas absorb or liberate heat?
Problem 27a
A monatomic ideal gas that is initially at Pa and has a volume of m3 is compressed adiabatically to a volume of m3. What is the final pressure?
Problem 27c
A monatomic ideal gas that is initially at Pa and has a volume of m3 is compressed adiabatically to a volume of m3. What is the ratio of the final temperature of the gas to its initial temperature? Is the gas heated or cooled by this compression?
Problem 28
Five moles of monatomic ideal gas have initial pressure Pa and initial volume m3. While undergoing an adiabatic expansion, the gas does J of work. What is the final pressure of the gas after the expansion?
Problem 30a
A player bounces a basketball on the floor, compressing it to of its original volume. The air (assume it is essentially N2 gas) inside the ball is originally at °C and atm. The ball's inside diameter is cm. What temperature does the air in the ball reach at its maximum compression? Assume the compression is adiabatic and treat the gas as ideal.
Problem 31
On a warm summer day, a large mass of air (atmospheric pressure Pa) is heated by the ground to °C and then begins to rise through the cooler surrounding air. (This can be treated approximately as an adiabatic process; why?) Calculate the temperature of the air mass when it has risen to a level at which atmospheric pressure is only Pa. Assume that air is an ideal gas, with . (This rate of cooling for dry, rising air, corresponding to roughly C° per m of altitude, is called the dry adiabatic lapse rate.)
Problem 32b
A cylinder contains 0.100 mol of an ideal monatomic gas. Initially the gas is at Pa and occupies a volume of m3. If the gas is allowed to expand to twice the initial volume, find the final temperature (in kelvins) and pressure of the gas if the expansion is (i) isothermal; (ii) isobaric; (iii) adiabatic.