Back
BackProblem 1
An electron is moving as a free particle in the -direction with momentum that has magnitude kg-m/s. What is the one-dimensional time-dependent wave function of the electron?
Problem 2ab
A free particle moving in one dimension has wave function where and are positive real constants.
(a) At , what are the two smallest positive values of for which the probability function is a maximum?
(b) Repeat part (a) for time .
Problem 3
An electron is moving as a free particle in the -direction with momentum that has magnitude kg*m/s. Let . At , the probability distribution function has a maximum at .
(a) What is the smallest positive value of for which the probability distribution function has a maximum at time , where ?
(b) From your result in part (a), what is the average speed with which the probability distribution is moving in the -direction?
Problem 4
A particle is described by a wave function , where and are real, positive constants. If the value of is increased, what effect does this have on (a) the particle’s uncertainty in position and (b) the particle’s uncertainty in momentum? Explain your answers.
Problem 5
Consider a wave function given by , where and is a real constant.
(a) For what values of is there the highest probability of finding the particle described by this wave function? Explain.
(b) For which values of is the probability zero? Explain.
Problem 7
Let and be two solutions of Eq. () [] with energies and respectively, where . Is , where and are nonzero constants, a solution to Eq. ()? Explain your answer.
Problem 9ab
(a) Find the lowest energy level for a particle in a box if the particle is a billiard ball ( kg) and the box has a width of m, the size of a billiard table. (Assume that the billiard ball slides without friction rather than rolls; that is, ignore the rotational kinetic energy.)
(b) Since the energy in part (a) is all kinetic, to what speed does this correspond? How much time would it take at this speed for the ball to move from one side of the table to the other?
Problem 9cd
A particle in a box is a billiard ball ( kg) and the box has a width of m, the size of a billiard table. (Assume that the billiard ball slides without friction rather than rolls; that is, ignore the rotational kinetic energy.) What is the difference in energy between the and levels? Are quantum mechanical effects important for the game of billiards?
Problem 10
A proton is in a box of width . What must the width of the box be for the ground-level energy to be MeV, a typical value for the energy with which the particles in a nucleus are bound? Compare your result to the size of a nucleus — that is, on the order of m.
Problem 12
When a hydrogen atom undergoes a transition from the to the level, a photon with nm is emitted.
(a) If the atom is modeled as an electron in a one-dimensional box, what is the width of the box in order for the to transition to correspond to emission of a photon of this energy?
(b) For a box with the width calculated in part (a), what is the ground state energy? How does this correspond to the ground state energy of a hydrogen atom?
(c) Do you think a one-dimensional box is a good model for a hydrogen atom? Explain. (Hint: Compare the spacing between adjacent energy levels as a function of .)
Problem 14
An electron in a one-dimensional box has ground state energy eV. What is the wavelength of the photon absorbed when the electron makes a transition to the second excited state?
Problem 16
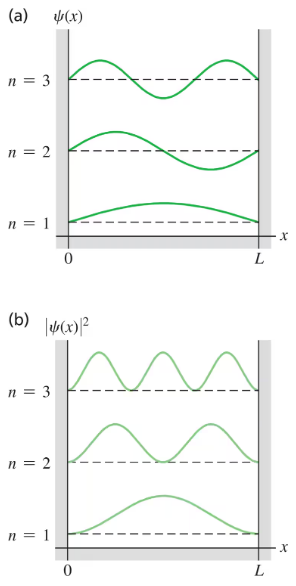
Recall that is the probability of finding the particle that has normalized wave function in the interval to . Consider a particle in a box with rigid walls at and . Let the particle be in the ground level and use as given in Eq. () where .
(a) For which values of , if any, in the range from to is the probability of finding the particle zero?
(b) For which values of is the probability highest?
(c) In parts (a) and (b) are your answers consistent with Fig. ? Explain.
Problem 18
(a) Find the excitation energy from the ground level to the third excited level for an electron confined to a box of width nm.
(b) The electron makes a transition from the to level by absorbing a photon. Calculate the wavelength of this photon.
Problem 19
An electron is in a box of width m. What are the de Broglie wavelength and the magnitude of the momentum of the electron if it is in (a) the level; (b) the level; (c) the level? In each case how does the wavelength compare to the width of the box?
Problem 27
(a) An electron with initial kinetic energy eV encounters a square barrier with height eV and width nm. What is the probability that the electron will tunnel through the barrier?
(b) A proton with the same kinetic energy encounters the same barrier. What is the probability that the proton will tunnel through the barrier?
Problem 29
An electron with initial kinetic energy eV encounters a barrier with height eV. What is the probability of tunneling if the width of the barrier is (a) nm and (b) nm?
Problem 30
An electron with initial kinetic energy eV encounters a barrier with height and width nm. What is the transmission coefficient if (a) eV; (b) eV; (c) eV?
Problem 38
While undergoing a transition from the to the energy level, a harmonic oscillator absorbs a photon of wavelength m. What is the wavelength of the absorbed photon when this oscillator undergoes a transition (a) from the to the energy level and (b) from the to the energy level?
(c) What is the value of , the angular oscillation frequency of the corresponding Newtonian oscillator?
Problem 39
For the ground level of a harmonic oscillator, . Do a similar analysis for an excited level that has quantum number . How does the uncertainty product depend on ?
Problem 40.20
An electron is in a box of width 3.0*10^-10 m. What are the de Broglie wavelength and the magnitude of the momentum of the electron if it is in (a) the n = 1 level; (b) the n = 2 level; (c) the n = 3 level? In each case how does the wavelength compare to the width of the box?
Problem 40.2c
A free particle moving in one dimension has wave function ψ(x,t) = A[e^i(kx-ωt) -e^i(2kx-4ωt)] where k and v are positive real constants. (c) Calculate v_av as the distance the maxima have moved divided by the elapsed time.