Back
BackProblem 48
A typical nuclear reactor generates 1000 MW (1000 MJ/s) of electric energy. In doing so, it produces 2000 MW of 'waste heat' that must be removed from the reactor to keep it from melting down. Many reactors are sited next to large bodies of water so that they can use the water for cooling. Consider a reactor where the intake water is at 18°C. State regulations limit the temperature of the output water to 30°C so as not to harm aquatic organisms. How many liters of cooling water have to be pumped through the reactor each minute?
Problem 49a
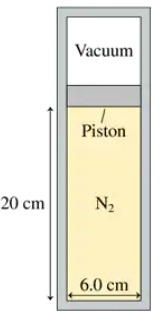
A 6.0-cm-diameter cylinder of nitrogen gas has a 4.0-cm-thick movable copper piston. The cylinder is oriented vertically, as shown in FIGURE P19.49, and the air above the piston is evacuated. When the gas temperature is 20°C, the piston floats 20 cm above the bottom of the cylinder. What is the gas pressure?
Problem 49c
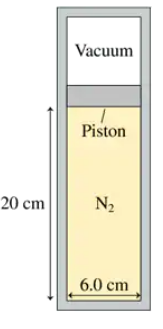
A 6.0-cm-diameter cylinder of nitrogen gas has a 4.0-cm-thick movable copper piston. The cylinder is oriented vertically, as shown in FIGURE P19.49, and the air above the piston is evacuated. When the gas temperature is 20°C, the piston floats 20 cm above the bottom of the cylinder. What is the new equilibrium temperature of the gas?
Problem 50b
2.0 mol of gas are at 30 °C and a pressure of 1.5 atm. How much work must be done on the gas to compress it to one third of its initial volume at constant pressure?
Problem 51
0.25 mol of a gas are compressed at a constant pressure of 250 kPa from 6000 cm3 to 2000 cm3, then expanded at a constant temperature back to 6000 cm3. What is the net work done on the gas?
Problem 52a
An ideal-gas process is described by p=cV1/2, where c is a constant. Find an expression for the work done on the gas in this process as the volume changes from V1 to V2.
Problem 52b
An ideal-gas process is described by p=cV1/2, where c is a constant. 0.033 mol of gas at an initial temperature of 150°C is compressed, using this process, from 300 cm3 to 200 cm3. How much work is done on the gas?
Problem 53b
A 10-cm-diameter cylinder contains argon gas at 10 atm pressure and a temperature of 50°C. A piston can slide in and out of the cylinder. The cylinder's initial length is 20 cm. 2500 J of heat are transferred to the gas, causing the gas to expand at constant pressure. What are the final length of the cylinder?
Problem 56a
n moles of an ideal gas at temperature T1 and volume V1 expand isothermally until the volume has doubled. In terms of n, T1, and V1, what is the final temperature?
Problem 56b
n moles of an ideal gas at temperature T1 and volume V1 expand isothermally until the volume has doubled. In terms of n, T1, and V1, what are the work done on the gas?
Problem 56c
n moles of an ideal gas at temperature T1 and volume V1 expand isothermally until the volume has doubled. In terms of n, T1, and V1, what are the heat energy transferred to the gas?
Problem 57a
5.0 g of nitrogen gas at 20°C and an initial pressure of 3.0 atm undergo an isobaric expansion until the volume has tripled. What are the gas volume and temperature after the expansion?
Problem 57b
5.0 g of nitrogen gas at 20°C and an initial pressure of 3.0 atm undergo an isobaric expansion until the volume has tripled. How much heat energy is transferred to the gas to cause this expansion?
Problem 57c
5.0 g of nitrogen gas at 20°C and an initial pressure of 3.0 atm undergo an isobaric expansion until the volume has tripled. What is the gas pressure after the decrease?
Problem 57d
5.0 g of nitrogen gas at 20°C and an initial pressure of 3.0 atm undergo an isobaric expansion until the volume has tripled. What amount of heat energy is transferred from the gas as its pressure decreases?
Problem 58
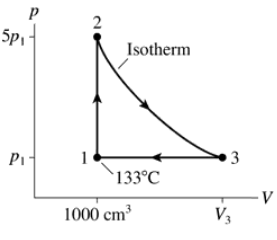
0.10 mol of nitrogen gas follow the two processes shown in FIGURE P19.58. How much heat is required for each?
Problem 61a
Two cylinders each contain 0.10 mol of a diatomic gas at 300 K and a pressure of 3.0 atm. Cylinder A expands isothermally and cylinder B expands adiabatically until the pressure of each is 1.0 atm. What are the final temperature and volume of each?
Problem 62b
FIGURE P19.62 shows a thermodynamic process followed by 120 mg of helium. How much work is done on the gas during each of the three segments?
Problem 62c
FIGURE P19.62 shows a thermodynamic process followed by 120 mg of helium. How much heat energy is transferred to or from the gas during each of the three segments?
Problem 64d
14 g of nitrogen gas at STP are adiabatically compressed to a pressure of 20 atm. What is the compression ratio Vmax/Vmin?
Problem 65a
14 g of nitrogen gas at STP are pressurized in an isochoric process to a pressure of 20 atm. What are the final temperature?
Problem 65d
A monatomic gas is adiabatically compressed to 1/8 of its initial volume. Does each of the following quantities change? If so, does it increase or decrease, and by what factor? If not, why not? The molar specific heat at constant volume.
Problem 67a
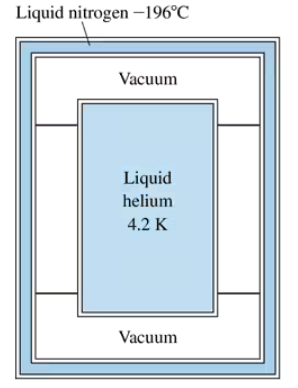
Liquid helium, with a boiling point of 4.2 K, is used in ultralow-temperature experiments and also for cooling the superconducting magnets used in MRI imaging in medicine. Storing liquid helium so far below room temperature is a challenge because even a small 'heat leak' will boil the helium away. A standard helium dewar, shown in FIGURE P19.67, has an inner stainless-steel cylinder filled with liquid helium surrounded by an outer cylindrical shell filled with liquid nitrogen at –196°C. The space between is a vacuum. The small structural supports have very low thermal conductivity, so you can assume that radiation is the only heat transfer between the helium and its surroundings. Suppose the helium cylinder is 16 cm in diameter and 30 cm tall and that all walls have an emissivity of 0.25. The density of liquid helium is 125 kg/m3 and its heat of vaporization is 2.1×104 J/kg. What is the mass of helium in the filled cylinder?
Problem 72
Most stars are main-sequence stars, a group of stars for which size, mass, surface temperature, and radiated power are closely related. The sun, for instance, is a yellow main-sequence star with a surface temperature of 5800 K. For a main-sequence star whose mass M is more than twice that of the sun, the total radiated power, relative to the sun, is approximately P/Psun=1.5(M/Msun)3.5. The star Regulus A is a bluish main-sequence star with mass 3.8Msun and radius 3.1Rsun. What is the surface temperature of Regulus A?
Problem 73
One cylinder in the diesel engine of a truck has an initial volume of 600 cm3. Air is admitted to the cylinder at 30°C and a pressure of 1.0 atm. The piston rod then does 400 J of work to rapidly compress the air. What are its final temperature and volume?
Problem 74
A flow-through electric water heater has a 20 kW electric heater inside an insulated 2.0-cm-diameter pipe so that water flowing through the pipe will have good thermal contact with the heater. Assume that all the heat energy is transferred to the water. Suppose the inlet water temperature is 12°C and the flow rate is 8.0 L/min (about that of a standard shower head). What is the outlet temperature?
Problem 75a
In Problems 75, 76, and 77 you are given the equation used to solve a problem. For each of these, you are to write a realistic problem for which this is the correct equation.
Problem 78
10 g of aluminum at 200°C and 20 g of copper are dropped into 50 cm3 of ethyl alcohol at 15°C. The temperature quickly comes to 25°C. What was the initial temperature of the copper?
Problem 79
A lava flow is threatening to engulf a small town. A 400-m-wide, 35-cm-thick tongue of 1200°C lava is advancing at the rate of 1.0 m per minute. The mayor devises a plan to stop the lava in its tracks by flying in large quantities of 20°C water and dousing it. The lava has density 2500 kg/m3, specific heat 1100 J/kg K, melting temperature 800°C, and heat of fusion 4.0×105 J/kg. How many liters of water per minute, at a minimum, will be needed to save the town?
Problem 80
FIGURE CP19.80 shows a thermodynamic process followed by 0.015 mol of hydrogen. How much heat energy is transferred to the gas?