Back
BackProblem 3
A hollow aluminum sphere with outer diameter 10.0 cm has a mass of 690 g. What is the sphere's inner diameter?
Problem 4
The nucleus of a uranium atom has a diameter of 1.5×10−14 m and a mass of 4.0×10−25 kg . What is the density of the nucleus?
Problem 5
How many atoms are in a 2.0 cm×2.0 cm×2.0 cm cube of aluminum?
Problem 7
An element in its solid phase has mass density 1750 kg/m3 and number density 4.39×1028 atoms/m3. What is the element's atomic mass number?
Problem 12
The lowest and highest natural temperatures ever recorded on earth are −129°F in Antarctica and 134°F in Death Valley. What are these temperatures in °C and in K?
Problem 13b
A demented scientist creates a new temperature scale, the 'Z scale.' He decides to call the boiling point of nitrogen 0°Z and the melting point of iron 1000°Z. Convert 500°Z to degrees Celsius and to kelvins.
Problem 14
At room temperature (20°C), a 5.0-cm-long brass rod is 20 μm too long to fit into a slot. To what temperature should you cool the rod so that it just barely fits?
Problem 17
A surveyor has a steel measuring tape that is calibrated to be 100.000 m long (i.e., accurate to ±1 mm) at 20°C. If she measures the distance between two stakes to be 65.175 m on a 3°C day, does she need to add or subtract a correction factor to get the true distance? How large, in mm, is the correction factor?
- Common outdoor thermometers are filled with red-colored ethyl alcohol. One thermometer has a 0.40-mm-diameter capillary tube attached to a 9.0-mm-diameter spherical bulb. On a 0°C morning, the column of alcohol stands 30 mm above the bulb. What is the temperature in °C when the column of alcohol stands 130 mm above the bulb? The expansion of the glass is much less than that of the alcohol and can be ignored.
Problem 18
Problem 20a
A cylinder contains nitrogen gas. A piston compresses the gas to half its initial volume. Afterward, has the mass density of the gas changed? If so, by what factor? If not, why not?
Problem 21a
A gas at 100°C fills volume V0. If the pressure is held constant, what is the volume if the Celsius temperature is doubled?
Problem 21b
A gas at 100°C fills volume V₀. If the pressure is held constant, what is the volume if the Kelvin temperature is doubled?
Problem 23
The total lung capacity of a typical adult is 5.0 L. Approximately 20% of the air is oxygen. At sea level and at a body temperature of 37°C, how many oxygen molecules do the lungs contain at the end of a strong inhalation?
Problem 24
The solar corona is a very hot atmosphere surrounding the visible surface of the sun. X-ray emissions from the corona show that its temperature is about 2×106 K. The gas pressure in the corona is about 0.03 Pa. Estimate the number density of particles in the solar corona.
Problem 25c
A 20-cm-diameter cylinder that is 40 cm long contains 50 g of oxygen gas at 20°C. What is the number density of the oxygen?
Problem 25d
A 20-cm-diameter cylinder that is 40 cm long contains 50 g of oxygen gas at 20°C. What is the reading of a pressure gauge attached to the tank?
Problem 27a
A gas with initial state variables p1, V1, and T1 is cooled in an isochoric process until p2 = (1/3)p1. What are V2?
Problem 28a
A gas with initial state variables p1, V1, and T1 expands isothermally until V2 = 2V1. What are T1?
Problem 28b
A gas with initial state variables p1, V1, and T1 expands isothermally until V2 = 2V1. What are p2?
Problem 31b
A 24-cm-diameter vertical cylinder is sealed at the top by a frictionless 20 kg piston. The piston is 84 cm above the bottom when the gas temperature is 303°C. The air above the piston is at 1.00 atm pressure. What will the height of the piston be if the temperature is lowered to 15°C?
Problem 32a
0.10 mol of argon gas is admitted to an evacuated 50 cm3 container at 20°C. The gas then undergoes an isochoric heating to a temperature of 300°C. What is the final pressure of the gas?
Problem 32b
0.10 mol of argon gas is admitted to an evacuated 50 cm3 container at 20°C. The gas then undergoes an isochoric heating to a temperature of 300°C. Show the process on a pV diagram. Include a proper scale on both axes.
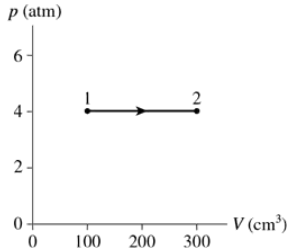
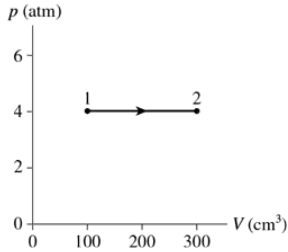
Problem 35a
A gas with an initial temperature of 900°C undergoes the process shown in FIGURE EX18.35. What type of process is this?
Problem 35c
A gas with an initial temperature of 900°C undergoes the process shown in FIGURE EX18.35. How many moles of gas are there?
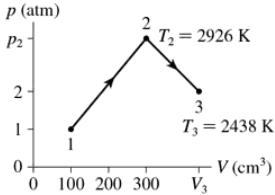
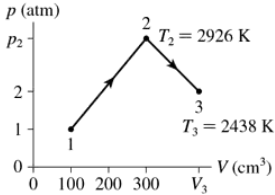
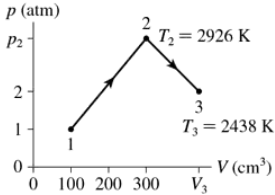
Problem 37a
0.0050 mol of gas undergoes the process 1→2→3 shown in FIGURE EX18.37. What are temperature T1?
Problem 37b
0.0050 mol of gas undergoes the process 1→2→3 shown in FIGURE EX18.37. What are pressure p2?
Problem 37c
0.0050 mol of gas undergoes the process 1→2→3 shown in FIGURE EX18.37. What is the volume V3?
Problem 40
A sealed container holds 3.2 g of oxygen at 1 atm pressure and 20°C. The gas first undergoes an isobaric process that doubles the absolute temperature, then an isothermal process that halves the pressure. What is the final volume of the gas in L?
Problem 41
The molecular mass of water (H₂O) is 18 u. How many protons are there in 1.0 L of liquid water?
Problem 42
The 828-m-tall Burj Khalifa in Dubai is the world's tallest building. It's essentially a steel building wrapped in exterior paneling and glass. During construction, when the beams were exposed to the elements, the building was 36 cm taller on the hottest afternoon of the year than on the coldest morning. By how much did the temperature vary throughout the year?