Back
BackProblem 2a
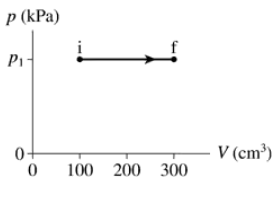
–60 J of work are done on the gas in the process shown in FIGURE EX19.2. What is p₁ in kPa?
Problem 3
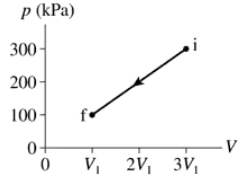
80 J of work are done on the gas in the process shown in FIGURE EX19.3. What is V1 in cm3?
Problem 7
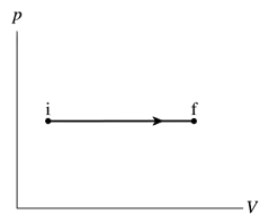
Draw a first-law bar chart (see Figure 19.12) for the gas process in FIGURE EX19.7.
Problem 11
500 J of heat energy are transferred to a gas during a process in which the gas expands at constant pressure from 400 cm3 to 800 cm3. The gas's thermal energy increases by 300 J during this process. What is the gas pressure?
Problem 12a
A rapidly spinning paddle wheel raises the temperature of 200 mL of water from 21°C to 25°C. How much heat is transferred?
Problem 13
How much heat energy must be added to a 6.0-cm-diameter copper sphere to raise its temperature from −50°C to 150°C?
Problem 16
What is the maximum mass of ethyl alcohol you could boil with 1000 J of heat, starting from 20°C?
Problem 17
One way you keep from overheating is by perspiring. Evaporation—a phase change—requires heat, and the heat energy is removed from your body. Evaporation is much like boiling, only water's heat of vaporization at 35°C is a somewhat larger 24×105 J/kg because at lower temperatures more energy is required to break the molecular bonds. Very strenuous activity can cause an adult human to produce 30 g of perspiration per minute. If all the perspiration evaporates, rather than dripping off, at what rate (in J/s) is it possible to exhaust heat by perspiring?
Problem 19a
Two cars collide head-on while each is traveling at 80 km/h. Suppose all their kinetic energy is transformed into the thermal energy of the wrecks. What is the temperature increase of each car? You can assume that each car's specific heat is that of iron.
Problem 20d
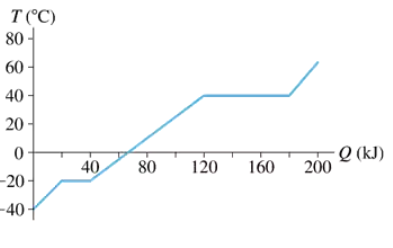
An experiment measures the temperature of a 500 g substance while steadily supplying heat to it. FIGURE EX19.20 shows the results of the experiment. What are the heats of fusion and vaporization?
Problem 21a
A 750 g aluminum pan is removed from the stove and plunged into a sink filled with 10.0 L of water at 20.0°C . The water temperature quickly rises to 24.0°C. What was the initial temperature of the pan in °C and in °F?
Problem 22
30 g of copper pellets are removed from a 300°C oven and immediately dropped into 100 mL of water at 20°C in an insulated cup. What will the new water temperature be?
Problem 24
A 65 cm3 block of iron is removed from an 800°C furnace and immediately dropped into 200 mL of 20°C water. What fraction of the water boils away?
Problem 25
10 g of steam at the boiling point are combined with 50 g of ice at the freezing point. What is the final temperature of the system?
Problem 26
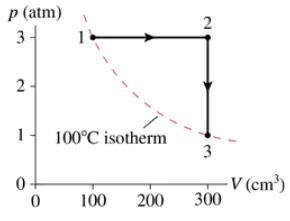
A monatomic gas follows the process 1→2→3 shown in FIGURE EX19.26. How much heat is needed for (a) process 1→2 and (b) process 2→3?
Problem 28a
A container holds 1.0 g of oxygen at a pressure of 8.0 atm. How much heat is required to increase the temperature by 100°C at constant pressure?
Problem 28b
A container holds 1.0 g of oxygen at a pressure of 8.0 atm. How much will the temperature increase if this amount of heat energy is transferred to the gas at constant volume?
Problem 30a
A gas cylinder holds 0.10 mol of O₂ at 150°C and a pressure of 3.0 atm. The gas expands adiabatically until the pressure is halved. What are the final volume?
Problem 30b
A gas cylinder holds 0.10 mol of O₂ at 150°C and a pressure of 3.0 atm. The gas expands adiabatically until the pressure is halved. What are the final temperature?
Problem 31a
The volume of a gas is halved during an adiabatic compression that increases the pressure by a factor of 2.5. What is the specific heat ratio γ?
Problem 31b
The volume of a gas is halved during an adiabatic compression that increases the pressure by a factor of 2.5. By what factor does the temperature increase?
Problem 32a
A gas cylinder holds 0.10 mol of O₂ at 150°C and a pressure of 3.0 atm. The gas expands adiabatically until the volume is doubled. What are the final pressure?
Problem 34
The ends of a 20-cm-long, 2.0-cm-diameter rod are maintained at 0°C and 100°C by immersion in an ice-water bath and boiling water. Heat is conducted through the rod at 4.5×104 J per hour. Of what material is the rod made?
Problem 35
You are boiling pasta and absentmindedly grab a copper stirring spoon rather than your wooden spoon. The copper spoon has a 20 mm ×1.5 mm rectangular cross section, and the distance from the boiling water to your 35°C hand is 18 cm. How long does it take the spoon to transfer 25 J of energy to your hand?
Problem 38
A 5.0-m-diameter garden pond is 30 cm deep. Solar energy is incident on the pond at an average rate of 400 W/m2. If the water absorbs all the solar energy and does not exchange energy with its surroundings, how many hours will it take to warm from 15°C to 25°C?
Problem 39
A 5.0 g ice cube at −20°C is in a rigid, sealed container from which all the air has been evacuated. How much heat is required to change this ice cube into steam at 200°C? Steam has cV = 1500 J/kg K and cP = 1960 J/kg K.
Problem 40
The burner on an electric stove has a power output of 2.0 kW. A 750 g stainless steel teakettle is filled with 20°C water and placed on the already hot burner. If it takes 3.0 min for the water to reach a boil, what volume of water, in cm3, was in the kettle? Stainless steel is mostly iron, so you can assume its specific heat is that of iron.
Problem 41
When air is inhaled, it quickly becomes saturated with water vapor as it passes through the moist airways. Consequently, an adult human exhales about 25 mg of evaporated water with each breath. Evaporation—a phase change—requires heat, and the heat energy is removed from your body. Evaporation is much like boiling, only water's heat of vaporization at 35°C is a somewhat larger 24×105 J/kg because at lower temperatures more energy is required to break the molecular bonds. At 12 breaths/min, on a dry day when the inhaled air has almost no water content, what is the body's rate of energy loss (in J/s) due to exhaled water? (For comparison, the energy loss from radiation, usually the largest loss on a cool day, is about 100 J/s.)
Problem 43
512 g of an unknown metal at a temperature of 15°C is dropped into a 100 g aluminum container holding 325 g of water at 98°C. A short time later, the container of water and metal stabilizes at a new temperature of 78°C. Identify the metal.
Problem 45
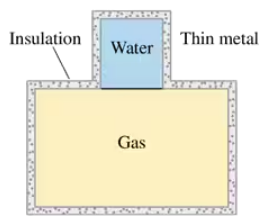
The beaker in FIGURE P19.45, with a thin metal bottom, is filled with 20 g of water at 20°C. It is brought into good thermal contact with a 4000 cm3 container holding 0.40 mol of a monatomic gas at 10 atm pressure. Both containers are well insulated from their surroundings. What is the gas pressure after a long time has elapsed? You can assume that the containers themselves are nearly massless and do not affect the outcome.