Back
BackProblem 44a
The semiconductor industry manufactures integrated circuits in large vacuum chambers where the pressure is 1.0×10-10 mm of Hg. What fraction is this of atmospheric pressure?
Problem 44b
The semiconductor industry manufactures integrated circuits in large vacuum chambers where the pressure is 1.0×10-10 mm of Hg. At T=20°C, how many molecules are in a cylindrical chamber 40 cm in diameter and 30 cm tall?
Problem 45
A 6.0-cm-diameter, 10-cm-long cylinder contains 100 mg of oxygen (O₂) at a pressure less than 1 atm. The cap on one end of the cylinder is held in place only by the pressure of the air. One day when the atmospheric pressure is 100 kPa, it takes a 184 N force to pull the cap off. What is the temperature of the gas?
Problem 47
On average, each person in the industrialized world is responsible for the emission of 10,000 kg of carbon dioxide (CO₂) every year. This includes CO₂ that you generate directly, by burning fossil fuels to operate your car or your furnace, as well as CO₂ generated on your behalf by electric generating stations and manufacturing plants. CO₂ is a greenhouse gas that contributes to global warming. If you were to store your yearly CO₂ emissions in a cube at STP, how long would each edge of the cube be?
Problem 49
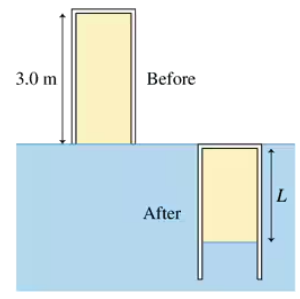
The 3.0-m-long pipe in FIGURE P18.49 is closed at the top end. It is slowly pushed straight down into the water until the top end of the pipe is level with the water's surface. What is the length L of the trapped volume of air?
Problem 50a
A diving bell is a 3.0-m-tall cylinder closed at the upper end but open at the lower end. The temperature of the air in the bell is 20°C. The bell is lowered into the ocean until its lower end is 100 m deep. The temperature at that depth is 10°C. How high does the water rise in the bell after enough time has passed for the air inside to reach thermal equilibrium?
Problem 51a
An electric generating plant boils water to produce high-pressure steam. The steam spins a turbine that is connected to the generator. How many liters of water must be boiled to fill a 5.0 m3 boiler with 50 atm of steam at 400°C?
Problem 52
On a cool morning, when the temperature is 15°C, you measure the pressure in your car tires to be 30 psi. After driving 20 mi on the freeway, the temperature of your tires is 45°C . What pressure will your tire gauge now show?
Problem 53
A 10-cm-diameter, 40-cm-tall gas cylinder, sealed at the top by a frictionless 50 kg piston, is surrounded by a bath of 20°C water. Then 50 kg of sand is slowly poured onto the top of the piston, where it stays. Afterward, what is the height of the piston?
Problem 55
The interior of a Boeing 737-800 can be modeled as a 32-m-long, 3.7-m-diameter cylinder. The air inside, at cruising altitude, is 20°C at a pressure of 82 kPa. What volume of outside air, at −40°C and a pressure of 23 kPa, must be drawn in, heated, and compressed to fill the plane?
Problem 57a
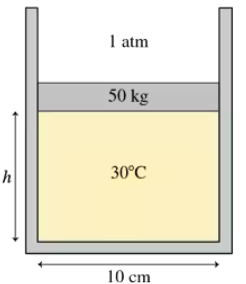
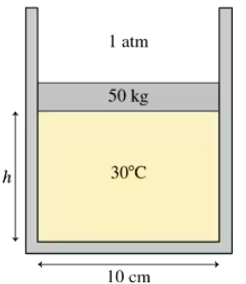
The 50 kg circular piston shown in FIGURE P18.57 floats on 0.12 mol of compressed air. What is the piston height h if the temperature is 30°C?
Problem 57b
The 50 kg circular piston shown in FIGURE P18.57 floats on 0.12 mol of compressed air. How far does the piston move if the temperature is increased by 100°C?
Problem 59
A diver 50 m deep in 10°C fresh water exhales a 1.0-cm-diameter bubble. What is the bubble's diameter just as it reaches the surface of the lake, where the water temperature is 20°C?
Problem 59b
In Problems and you are given the equation(s) used to solve a problem. For each of these, you are to draw a pV diagram.
Problem 64a
10 g of dry ice (solid CO₂) is placed in a 10,000 cm3 container, then all the air is quickly pumped out and the container sealed. The container is warmed to 0°C, a temperature at which CO₂ is a gas. What is the gas pressure? Give your answer in atm. The gas then undergoes an isothermal compression until the pressure is 3.0 atm, immediately followed by an isobaric compression until the volume is 1000 cm3.
Problem 65b
A container of gas at 2.0 atm pressure and 127°C is compressed at constant temperature until the volume is halved. It is then further compressed at constant pressure until the volume is halved again. Show this process on a pV diagram.
Problem 66a
Five grams of nitrogen gas at an initial pressure of 3.0 atm and at 20°C undergo an isobaric expansion until the volume has tripled. What is the gas volume after the expansion?
Problem 66b
Five grams of nitrogen gas at an initial pressure of 3.0 atm and at 20°C undergo an isobaric expansion until the volume has tripled. What is the gas temperature after the expansion (in °C)? The gas pressure is then decreased at constant volume until the original temperature is reached.
Problem 67a
In Problems and you are given the equation(s) used to solve a problem. For each of these, you are to write a realistic problem for which this is the correct equation(s).
Problem 68a
In Problems and you are given the equation(s) used to solve a problem. For each of these, you are to write a realistic problem for which this is the correct equation(s).
Problem 72
An inflated bicycle inner tube is 2.2 cm in diameter and 200 cm in circumference. A small leak causes the gauge pressure to decrease from 110 psi to 80 psi on a day when the temperature is 20°C. What mass of air is lost? Assume the air is pure nitrogen.
Problem 73
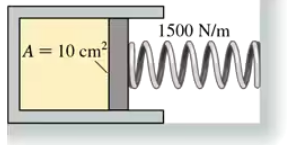
The cylinder in FIGURE CP18.73 has a moveable piston attached to a spring. The cylinder's cross-section area is 10 cm2, it contains 0.0040 mol of gas, and the spring constant is 1500 N/m. At 20°C the spring is neither compressed nor stretched. How far is the spring compressed if the gas temperature is raised to 100°C?
Problem 74b
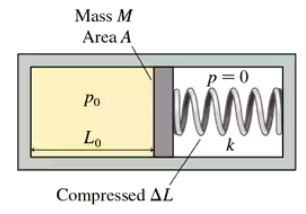
The closed cylinder of FIGURE CP18.74 has a tight-fitting but frictionless piston of mass M. The piston is in equilibrium when the left chamber has pressure p₀ and length L₀ while the spring on the right is compressed by ΔL. Suppose the piston is moved a small distance x to the right. Find an expression for the net force (Fₓ)net on the piston. Assume all motions are slow enough for the gas to remain at the same temperature as its surroundings.