3. Acids and Bases
Acid Base Equilibrium
Practice this topic
- Multiple ChoiceWhat is the approximate Keq for the following reaction?
- Textbook Question
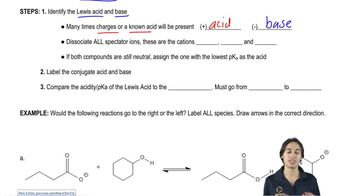
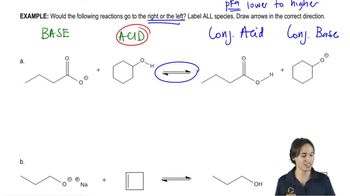
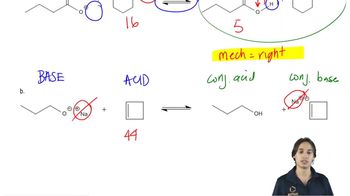
Consider the following proposed Brønsted–Lowry acid–base reactions. In each case, draw the products of a transfer of the most acidic proton on the acid to the most basic site on the base. Use Appendix 4 to find or estimate the pKa values for the acids and the pKb values for the bases. Then determine which side of the reaction is favored, either reactants or products.
(a)
(b)
- Textbook Question
The Ka of phenylacetic acid is 5.2 × 10−5, and the pKa of propionic acid is 4.87.
c. Predict whether the following equilibrium will favor the reactants or the products.
- Textbook Question
Write equations for the following acid–base reactions. Use the information in Table 2-2 or Appendix 4 to predict whether the equilibrium will favor the reactants or the products.
a. HCOOH + –CN
b. CH3COO– + CH3OH
c. (CH3)2CHOH + NaNH2
- Textbook Question
For each of the following reactions, suggest which solvent(s) would be compatible with the acids and bases involved. (We will ignore any other possible reactions for now.) Your choices of solvents are pentane, diethyl ether, ethanol, water, and ammonia. Refer to Appendix 4 for any needed values of pKa, or estimate them.
a. CH3Li + H—C≡C—H → CH4 + H—C≡CLi
b. CH3Li + (CH3)3C—OH → CH4 + (CH3)3C—OLi
- Multiple ChoiceIn the context of acid-base equilibrium, which of the following factors can disrupt the Hardy-Weinberg equilibrium?
- Multiple ChoiceWhich of the following statements best explains why scientists study acid-base equilibrium in organic chemistry?
- Multiple ChoiceWhich of the following statements is true regarding the effect of enzyme phosphorylation on acid-base equilibrium?