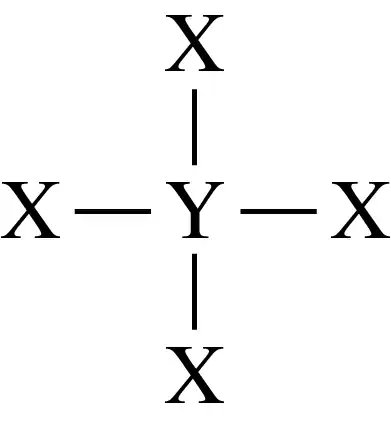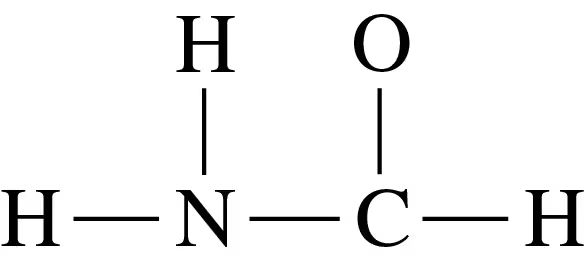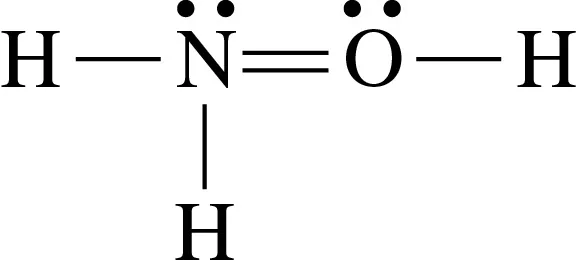Back
BackProblem 99
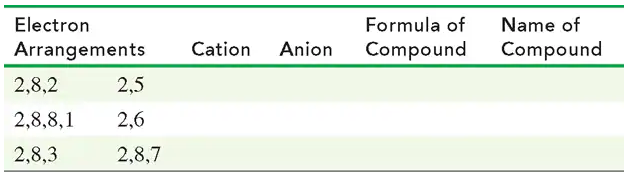
Using each of the following electron arrangements, give the formulas for the cation and anion that form, the formula for the compound they form, and its name.
Problem 102c
State the number of valence electrons, bonding pairs, and lone pairs in each of the following Lewis structures:
c.
Problem 103c
Match each of the Lewis structures (a to c) with the correct diagram (1 to 3) of its shape, and name the shape; indicate if each molecule is polar or nonpolar. Assume X and Y are nonmetals and all bonds are polar covalent.
<IMAGE>
c.
Problem 104a
Match each of the formulas (a to c) with the correct diagram (1 to 3) of its shape, and name the shape; indicate if each molecule is polar or nonpolar.
<IMAGE>
a. PBr3
Problem 105d
Consider the following bonds: Ca and O, C and O, K and O, O and O, and N and O.
d. Arrange the covalent bonds in order of decreasing polarity.
Problem 111b
Consider an ion with the symbol X2+ formed from a representative element.
b. What is the Lewis symbol of the element?
Problem 111c
Consider an ion with the symbol X2+ formed from a representative element.
c. If X is in Period 3, what is the element?
Problem 111d
Consider an ion with the symbol X2+ formed from a representative element.
d. What is the formula of the compound formed from X and the nitride ion?
Problem 112b
Consider an ion with the symbol Y3- formed from a representative element.
b. What is the Lewis symbol of the element?
Problem 112c
Consider an ion with the symbol Y3- formed from a representative element.
c. If Y is in Period 3, what is the element?
Problem 113a
One of the ions of tin is tin(IV).
a. What is the symbol for this ion?
Problem 113b
One of the ions of tin is tin(IV).
b. How many protons and electrons are in the ion?
Problem 127b
Draw the Lewis structure for each of the following:
b. H2NOH (N is the central atom)
Problem 128a
Draw the Lewis structure for each of the following:
a. H3COCH3 (the atoms are in the order C O C)
Problem 131c
Select the more polar bond in each of the following pairs:
c. Br―Cl or S―Cl
Problem 133a
Show the dipole arrow for each of the following bonds:
a. Si―Cl
Problem 135a
Calculate the electronegativity difference and classify each of the following bonds as nonpolar covalent, polar covalent, or ionic:
a. Si and Cl
Problem 135b
Calculate the electronegativity difference and classify each of the following bonds as nonpolar covalent, polar covalent, or ionic:
b. C and C
Problem 135c
Calculate the electronegativity difference and classify each of the following bonds as nonpolar covalent, polar covalent, or ionic:
c. Na and Cl
Problem 141a
Predict the shape and polarity of each of the following molecules, which have polar covalent bonds:
a. A central atom with three identical bonded atoms and one lone pair.
Problem 149a
Identify the group number in the periodic table of X, a representative element, in each of the following ionic compounds:
a. XCl3
Problem 149b
Identify the group number in the periodic table of X, a representative element, in each of the following ionic compounds:
b. Al2X3
Problem 150b
Identify the group number in the periodic table of X, a representative element, in each of the following ionic compounds:
b. X2SO3
Problem 151a
Classify each of the following as ionic or molecular, and name each:
a. Li2HPO4
Problem 152b
Classify each of the following as ionic or molecular, and name each:
b. Cl2O7
Problem 153a
Complete the Lewis structure for each of the following:
a.
Problem 154c
Identify the errors in each of the following Lewis structures and draw the correct formula:
c.