Where does fusion occur naturally?
Ch.5 Nuclear Chemistry

Timberlake13th EditionChemistry: An Introduction to General, Organic, and Biological ChemistryISBN: 9780134421353Not the one you use?Change textbook
Chapter 5, Problem 66a
Write the balanced nuclear equation for each of the following:
a. Polonium-210 decays to give lead-206.
 Verified step by step guidance
Verified step by step guidance1
Identify the type of nuclear decay occurring. Polonium-210 (Po-210) decays to lead-206 (Pb-206), which suggests an alpha decay process. In alpha decay, an alpha particle (⁴₂He) is emitted.
Write the general form of an alpha decay equation:
Determine the atomic number and mass number of the parent isotope (Po-210). Polonium has an atomic number of 84 and a mass number of 210. Represent it as:
Subtract the mass and atomic numbers of the alpha particle (⁴₂He) from the parent isotope to find the daughter isotope. The mass number decreases by 4 (210 - 4 = 206), and the atomic number decreases by 2 (84 - 2 = 82). The daughter isotope is lead-206 (Pb-206).
Write the balanced nuclear equation:

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
2mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Nuclear Decay
Nuclear decay is the process by which an unstable atomic nucleus loses energy by emitting radiation. This can occur in various forms, including alpha decay, beta decay, and gamma decay. In the case of Polonium-210, it undergoes alpha decay, where it emits an alpha particle (helium nucleus) and transforms into a different element, lead-206.
Recommended video:
Guided course
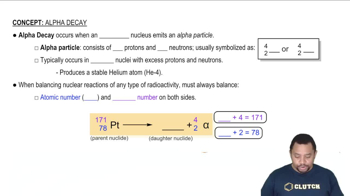
Alpha Decay
Balanced Nuclear Equation
A balanced nuclear equation represents the transformation of one element into another during a nuclear reaction, ensuring that the total number of protons and neutrons is conserved. In these equations, the atomic numbers and mass numbers of the reactants and products must be equal. This balance is crucial for accurately depicting the decay process and the resulting elements.
Recommended video:
Guided course
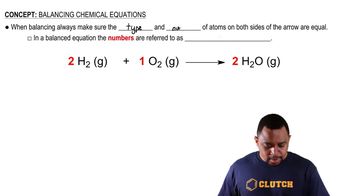
Balancing Chemical Equations (Simplified) Concept 1
Alpha Particle
An alpha particle is a type of radiation consisting of two protons and two neutrons, essentially a helium nucleus. It is emitted during alpha decay, a common form of radioactive decay for heavy elements like Polonium-210. The emission of an alpha particle reduces the atomic number of the original element by two and the mass number by four, leading to the formation of a new element.
Recommended video:
Guided course

Alpha Decay Reaction Example
Related Practice
Textbook Question
Textbook Question
Write the balanced nuclear equation for each of the following:
e. In-113m (γ emission)
Textbook Question
Complete each of the following nuclear equations:
d. 23m12Mg → ? + 00γ
Textbook Question
Write the balanced nuclear equation for each of the following:
a. When two oxygen-16 atoms collide, one of the products is an alpha particle.
Textbook Question
What are the products in the fission of uranium-235 that make possible a nuclear chain reaction?
Textbook Question
All the elements beyond uranium, the transuranium elements, have been prepared by bombardment and are not naturally occurring elements. The first transuranium element neptunium, Np, was prepared by bombarding U-238 with neutrons to form a neptunium atom and a beta particle. Complete the following equation:
10n + 23892U →? + ?
