Trends in the Periodic Table
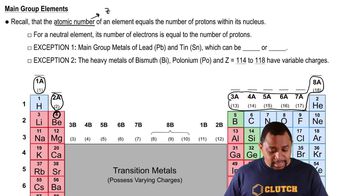
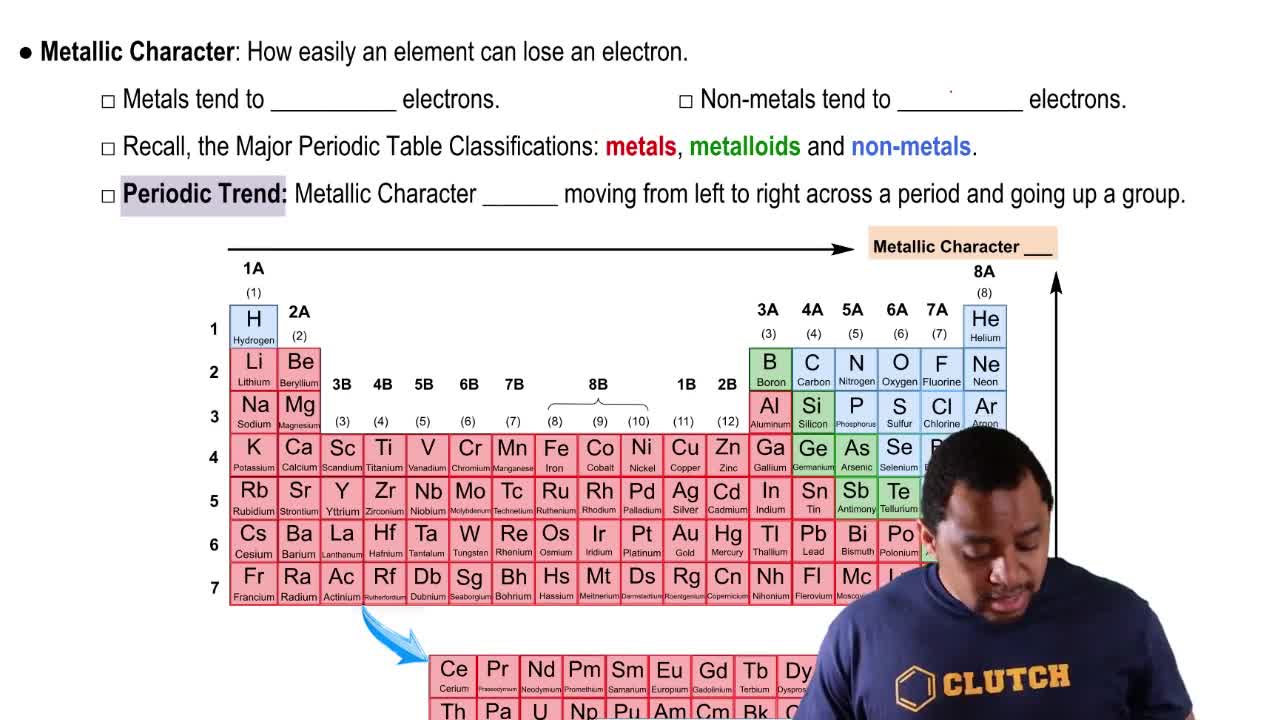
Trends in the periodic table, such as electronegativity, ionization energy, and metallic character, help predict the behavior of elements. For Group 4A, as you move from carbon to lead, the metallic character increases. Therefore, to identify the element with the least metallic character in this group, one must recognize that carbon, being the topmost element, exhibits the highest non-metallic character.

 Verified step by step guidance
Verified step by step guidance