Textbook Question
Stearic acid and linoleic acid each have 18 carbon atoms. Why does stearic acid melt at 69 °C but linoleic acid melts at –5 °C?

 Verified step by step guidance
Verified step by step guidance

Stearic acid and linoleic acid each have 18 carbon atoms. Why does stearic acid melt at 69 °C but linoleic acid melts at –5 °C?
Draw the line-angle formula for each of the following fatty acids:
a. palmitic acid
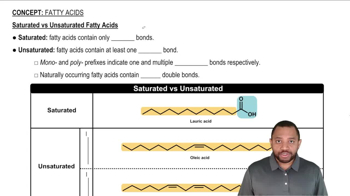
For each of the following fatty acids, give the shorthand notation for the number of carbon atoms and double bonds, and classify as saturated, monounsaturated, or polyunsaturated:
a. lauric acid
How does the structure of a fatty acid with a cis double bond differ from the structure of a fatty acid with a trans double bond?
How does the double bond influence the dispersion forces that can form between the hydrocarbon chains of fatty acids?
Compare the structures and functional groups of arachidonic acid and prostaglandin PGE1.