Identify the compound in each group that is most soluble in water. Explain.
b. pentane, 1-hexanol, propanoic acid

 Verified step by step guidance
Verified step by step guidance
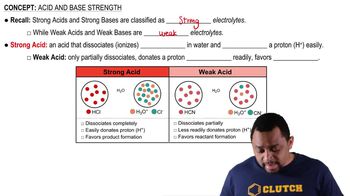
Identify the compound in each group that is most soluble in water. Explain.
b. pentane, 1-hexanol, propanoic acid
Identify the compound in each group that is most soluble in water. Explain.
b. ethanoic acid (acetic acid), hexanoic acid, octanoic acid
Write the balanced chemical equation for the dissociation of each of the following carboxylic acids in water:
a. pentanoic acid
Write the balanced chemical equation for the reaction of each of the following carboxylic acids with NaOH:
c. benzoic acid
Write the balanced chemical equation for the reaction of each of the following carboxylic acids with KOH:
b. 2-methylbutanoic acid
Write the IUPAC and common names, if any, of the carboxylate salts produced in problem 14.11
a. formic acid
b. 3-chloropropanoic acid
c. benzoic acid