Textbook Question
Give the IUPAC name for each of the following:
d.

 Timberlake 13th Edition
Timberlake 13th Edition Ch.12 Alcohols, Thiols, Ethers, Aldehydes, and Ketones
Ch.12 Alcohols, Thiols, Ethers, Aldehydes, and Ketones Problem 3c
Problem 3c Verified step by step guidance
Verified step by step guidance
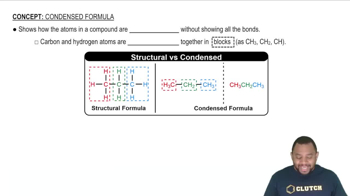
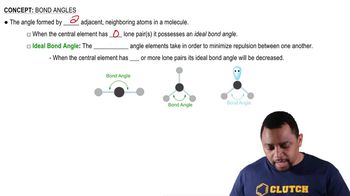
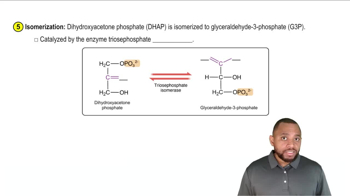
Give the IUPAC name for each of the following:
d.
Draw the condensed structural formula, or line-angle formula if cyclic, for each of the following:
a. propyl alcohol
Draw the condensed structural formula, or line-angle formula if cyclic, for each of the following:
b. 3-pentanethiol
Draw the condensed structural formula, or line-angle formula if cyclic, for each of the following:
d. 4-bromophenol
Draw the condensed structural formula, or line-angle formula if cyclic, for each of the following:
a. ethyl alcohol
Draw the condensed structural formula, or line-angle formula if cyclic, for each of the following:
c. 1-propanethiol