Using wedge-and-dash bonds, draw a cis and a trans stereoisomers for each of the following compounds:
(b) 1-bromo-2-ethylcyclopentane

 Verified step by step guidance
Verified step by step guidance
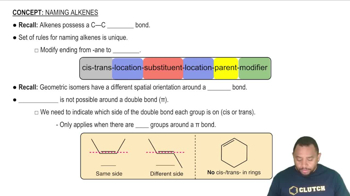

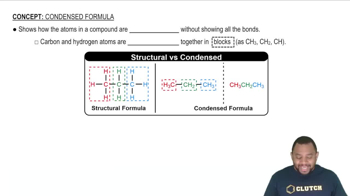
Using wedge-and-dash bonds, draw a cis and a trans stereoisomers for each of the following compounds:
(b) 1-bromo-2-ethylcyclopentane
Using wedge-and-dash bonds, draw both the cis and trans stereoisomers for each of the following compounds:
(b) 1,3-diethylcyclobutane
For each of the following compounds, indicate whether or not it can exist as cis–trans stereoisomers. If it can exist as the two isomers, draw both as a condensed structure.
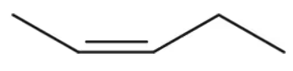
(a) H2C=CHCH2CH3
For each of the following compounds, indicate whether or not it can exist as cis–trans stereoisomers. If it can exist as the two isomers, draw both as a condensed structure.
(c)
The structure of vitamin A is shown in Problem 4.54. Is the double bond nearest the ring on the carbon chain cis or trans?
The vision process in animals involves the change of one double bond in retinal from its cis form to its trans form. The structures of cis and trans retinal are shown below. Label which is cis and which is trans.