A Freon leak in the air-conditioning system of an old car releases 38.0 g of CF2Cl2 per month. What mass of chlorine does this car emit into the atmosphere each year?
Ch.3 - Molecules and Compounds

Chapter 3, Problem 126
A metal (M) forms an oxide with the formula M2O. If the oxide contains 16.99% O by mass, what is the identity of the metal?
 Verified step by step guidance
Verified step by step guidance1
Calculate the molar mass of oxygen (O), which is approximately 16.00 g/mol.
Let the molar mass of the metal (M) be represented as x g/mol.
The molar mass of the oxide M2O is 2x + 16.00 g/mol.
Set up the equation for the mass percentage of oxygen: (16.00 / (2x + 16.00)) * 100 = 16.99.
Solve the equation for x to find the molar mass of the metal M.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
5mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Molar Mass and Percent Composition
Molar mass is the mass of one mole of a substance, typically expressed in grams per mole. Percent composition refers to the percentage by mass of each element in a compound. In this question, knowing the percent composition of oxygen in the oxide allows us to calculate the molar mass of the metal by using the formula for percent composition.
Recommended video:
Guided course

Mass Percent Calculation
Stoichiometry
Stoichiometry is the branch of chemistry that deals with the relationships between the quantities of reactants and products in chemical reactions. In this case, the stoichiometric ratio of the metal (M) to oxygen in the oxide M2O is crucial for determining the molar mass of the metal based on the given mass percentage of oxygen.
Recommended video:
Guided course

Stoichiometry Concept
Identifying Elements from Molar Mass
Once the molar mass of the metal is calculated, it can be compared to known molar masses of elements on the periodic table to identify the metal. Each element has a unique molar mass, and this comparison allows us to determine which metal corresponds to the calculated value based on the oxide's formula.
Recommended video:
Guided course
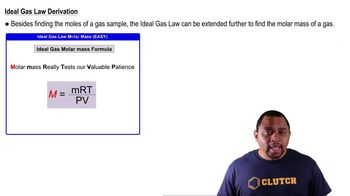
Molar Mass from Ideal Gas Law
Related Practice
Textbook Question
Textbook Question
Determine the chemical formula of each compound and then use it to calculate the mass percent composition of each constituent element. d. cobalt(II) bromide
Textbook Question
A metal (M) forms a compound with the formula MCl3. If the compound contains 65.57% Cl by mass, what is the identity of the metal?
2
views
Textbook Question
Fructose is a common sugar found in fruit. Elemental analysis of fructose gives the following mass percent composition: C 40.00%, H 6.72%, O 53.28%. The molar mass of fructose is 180.16 g/mol. Find the molecular formula of fructose.
Textbook Question
Combustion analysis of a 13.42-g sample of equilin (which contains only carbon, hydrogen, and oxygen) produces 39.61 g CO2 and 9.01 g H2O. The molar mass of equilin is 268.34 g/mol. Find its molecular formula.
1
views
