Determine the chemical formula of each compound and then use it to calculate the mass percent composition of each constituent element. a. potassium chromate b. lead(II) phosphate
Ch.3 - Molecules and Compounds

Chapter 3, Problem 123
A Freon leak in the air-conditioning system of an old car releases 38.0 g of CF2Cl2 per month. What mass of chlorine does this car emit into the atmosphere each year?
 Verified step by step guidance
Verified step by step guidance1
Determine the molar mass of CF_2Cl_2 by adding the atomic masses of its constituent atoms: C, F, and Cl.
Calculate the number of moles of CF_2Cl_2 released per month by dividing the mass released (38.0 g) by the molar mass of CF_2Cl_2.
Determine the number of moles of chlorine atoms in the moles of CF_2Cl_2, considering that each molecule of CF_2Cl_2 contains two chlorine atoms.
Calculate the mass of chlorine released per month by multiplying the moles of chlorine by the atomic mass of chlorine.
Calculate the total mass of chlorine released per year by multiplying the monthly chlorine mass by 12 (months in a year).

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
5mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Molar Mass Calculation
To determine the mass of chlorine emitted, one must first calculate the molar mass of CF2Cl2 (dichlorodifluoromethane). The molar mass is the sum of the atomic masses of all atoms in the molecule: carbon (C), fluorine (F), and chlorine (Cl). This calculation is essential for converting grams of CF2Cl2 to moles, which will then allow for the determination of the mass of chlorine released.
Recommended video:
Guided course

Molar Mass Calculation Example
Stoichiometry
Stoichiometry is the branch of chemistry that deals with the relationships between the quantities of reactants and products in a chemical reaction. In this case, it involves using the mole ratio from the chemical formula of CF2Cl2 to find out how many moles of chlorine are present in the given mass of CF2Cl2. This step is crucial for converting the amount of CF2Cl2 released into the corresponding mass of chlorine.
Recommended video:
Guided course

Stoichiometry Concept
Annual Emission Calculation
To find the annual emission of chlorine, one must multiply the monthly release of CF2Cl2 by 12 months. This calculation provides the total mass of CF2Cl2 released in a year, which can then be used to find the total mass of chlorine emitted. Understanding how to scale monthly emissions to an annual basis is important for environmental assessments and regulatory compliance.
Recommended video:
Guided course
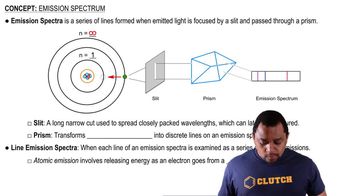
Emission Spectra
Related Practice
Textbook Question
Textbook Question
Determine the chemical formula of each compound and then use it to calculate the mass percent composition of each constituent element. c. sulfurous acid
Textbook Question
Determine the chemical formula of each compound and then use it to calculate the mass percent composition of each constituent element. c. nitrogen triiodide
Textbook Question
Determine the chemical formula of each compound and then use it to calculate the mass percent composition of each constituent element. d. cobalt(II) bromide
Textbook Question
A metal (M) forms a compound with the formula MCl3. If the compound contains 65.57% Cl by mass, what is the identity of the metal?
2
views
Textbook Question
A metal (M) forms an oxide with the formula M2O. If the oxide contains 16.99% O by mass, what is the identity of the metal?
2
views
